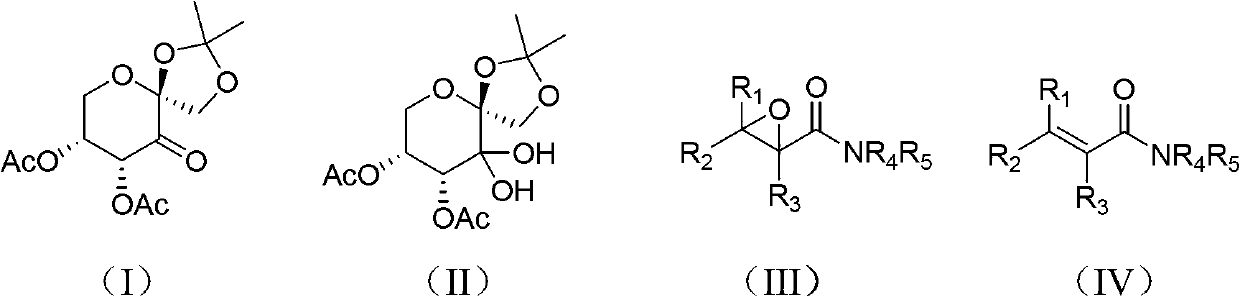
Preparation method of alpha,beta-epoxy amide compounds
A technology of amide compounds and compounds, applied in the direction of organic chemistry, etc., can solve the problems of incapable mass production, and achieve the effects of mild reaction conditions, simple operation, simple catalyst and operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
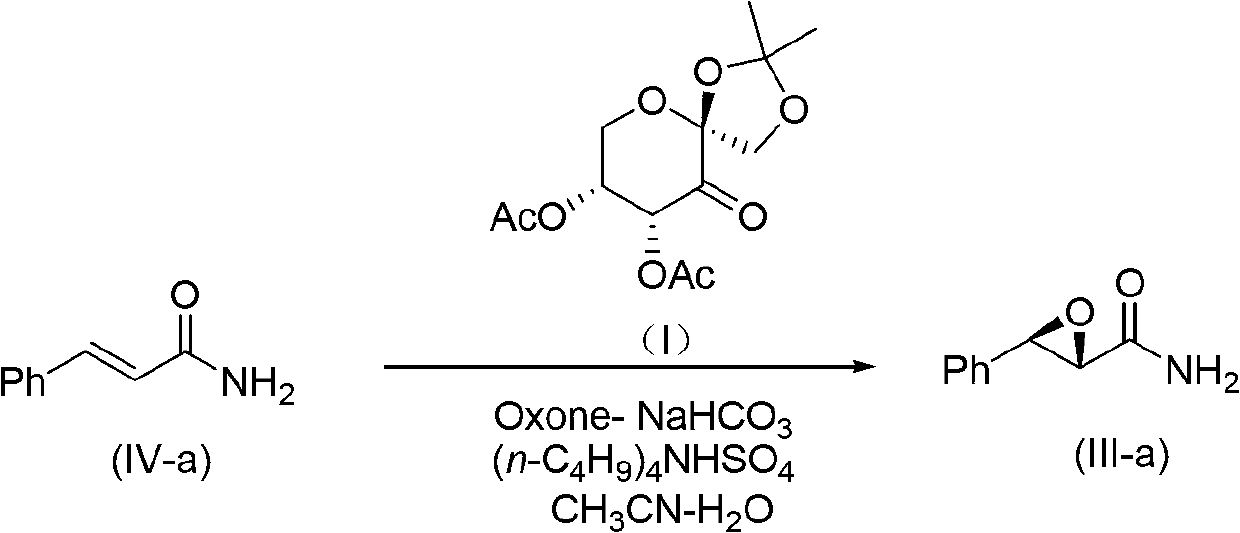
[0021] Example 1. Preparation of the compound ((+)-(2S, 3R)-3-Phenylglycidamide) represented by formula (III-a)
[0022]
[0023] The reaction equation is shown in the above formula, wherein, Ph is phenyl; Ac is acetyl;
[0024] Add trans-cinnamide (IV-a) (74.0mg, 0.5mmol) into a 100mL single-necked bottle, (n-C 4 h 9 ) 4 NHSO 4 (10.0mg, 0.03mmol, trans-cinnamide and (n-C 4 h 9 ) 4 NHSO 4 The mole fraction ratio is 1:0.06), add 2.5mL of acetonitrile to dissolve it and stir vigorously with polytetrafluoroethylene magnets (stirring speed will affect the conversion rate of the reaction); cool to 0°C (ice-water bath), add 1×10 -4 M Na 2 EDTA aqueous solution 2.5mL (added Na 2 The volume ratio of EDTA aqueous solution to the acetonitrile added to the reaction is 1:1); then add a small amount of Oxone (potassium hydrogen persulfate compound salt)-NaHCO that has been pulverized with a traditional Chinese medicine pulverizer 3 After adjusting the pH of the mixture to >7....
Embodiment 2
[0027] Example 2. Preparation of the compound ((+)-3-Phenyl-oxirane-2-carboxlic acid tert-butylamide) represented by formula (III-b)
[0028]
[0029] The reaction equation is as shown in the above formula, wherein, Ph is phenyl;
[0030] Add trans-cinnamoyl tert-butylamine (IV-b) (102.0mg, 0.5mmol) into a 100mL single-necked bottle, (n-C 4 h 9 ) 4 NHSO 4 (5.0mg, 0.015mmol, trans-cinnamamide tert-butylamine and (n-C 4 h 9 ) 4 NHSO 4 The molar fraction ratio is 1:0.03), add 2.5mL of acetonitrile to dissolve it and put it into polytetrafluoromagnetic submersible for vigorous stirring (stirring speed will affect the conversion rate of the reaction). Cool to 0°C (ice-water bath), add 1×10 -4 MNa 2 EDTA aqueous solution 1.25mL (added Na 2 The volume / part ratio of EDTA aqueous solution to the acetonitrile added in the reaction is 1:2). Then add a small amount of Oxone-NaHCO that has been pulverized with a traditional Chinese medicine pulverizer 3 After the mixture was...
Embodiment 3
[0033] Example 3. Preparation of the compound represented by formula (III-c) ((+)-(2S,3R)-3-Phenyl-oxirane-2-carboxlic acid benzylamide)
[0034]
[0035] The reaction equation is shown in the above formula, wherein Ph is phenyl; Bn is benzyl.
[0036] Add trans-cinnamoyl benzylamide (IV-c) (119.0mg, 0.5mmol) into a 100mL single-necked bottle, (n-C 4 h 9 ) 4 NHSO 4 (167mg, 0.5mmol; trans-cinnamoyl benzylamide and (n-C 4 h 9 ) 4 NHSO 4 The mole fraction ratio is 1:1), add 2.5mL of acetonitrile to dissolve it and put it into polytetrafluoromagnetic submersible to stir vigorously (stirring speed will affect the conversion rate of the reaction). Cool to 0°C (ice-water bath), add 1×10 -4 M Na 2 EDTA aqueous solution 2.5mL (added Na 2 The volume / part ratio of EDTA aqueous solution to the acetonitrile added in the reaction is 1:1). Then add a small amount of Oxone-NaHCO that has been pulverized with a traditional Chinese medicine pulverizer 3 After adjusting the pH of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com