Application of semiporphyrazine and its transition metal complexes as catalysts for lithium/thionyl chloride batteries
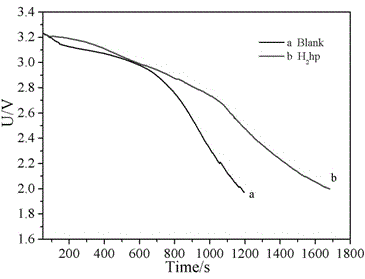
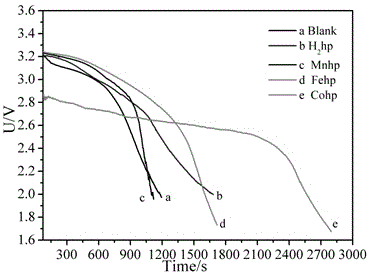
A thionyl chloride battery and transition metal technology, which is applied in battery electrodes, circuits, electrical components, etc., can solve problems that have not been reported in the literature, and achieve the effects of prolonging discharge time, improving discharge performance, and increasing discharge voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
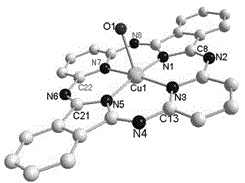
[0020] Embodiment 1: the synthesis of half porphyrazine
[0021] Add 6.00 g (40 mmol) of 1,3-diiminoisoindoline, 4.60 g (40 mmol) of 2,6-diaminopyridine, and 40.0 mL of n-butanol into a dry round-bottomed flask, at 120 °C The reaction was stirred for about 8 h, cooled to room temperature, and an orange-yellow solid was formed. The reaction solution was filtered under reduced pressure, and the obtained solid was washed with n-butanol, absolute ethanol, acetone, dichloromethane, and ether to obtain a crude product, which was recrystallized with nitrobenzene and dried in vacuum for 24 h to obtain a yellow crystal 3.00 g (H 2 hp), the yield was 34.0%, and the melting point was >300°C.
[0022] IR: 3261 (w, υ N-H ), 3020 (s, υ =C-H ), 1687, 1583, 1551 (s, υ C=C , υ C=N ), 1470, 1233, 1224 (s, υ C-N , υ C-C ), 767, 735 (s, δ =C-H ).
[0023] UV-vis (DMSO) λ max : 346 nm. HRMS (TOF MS ES-) calculated for C 26 h 16 N 8 : 440.1498, found: 441.1368 (C 26 h 16 N 8 +H + ...
Embodiment 2
[0025] Embodiment 2: the synthesis of half porphyrazine manganese complex
[0026] Add 0.40 g (0.91 mmol) of the semiporphyrazine ligand and 8.0 mL of N,N-dimethylformamide into a dry round-bottomed flask, stir at room temperature for 0.5 h, and then add 0.24 g (1.20 mmol) MnCl 2 4H 2 O, the reaction was stirred at 155°C for 3 h, cooled to room temperature, and a solid precipitate formed. The reaction solution was filtered under reduced pressure, and the resulting solid was washed with N,N-dimethylformamide, distilled water, absolute ethanol, acetone, and ether to obtain a crude product, which was purified with ethanol and dried in vacuo to obtain 0.35 g of a gray product ( Mnhp), the productive rate is 77.5%, melting point>300 ℃.
[0027] IR: 3010 (s, υ =C-H ), 1660, 1580, 1544 (s, υ C=C , υ C=N ), 1455, 1250, 1220 (s, υ C-N , υ C-C ), 760, 730 (s, δ =C-H ).
[0028] UV-vis (DMSO) λ max : 396 nm.
Embodiment 3
[0029] Embodiment 3: the synthesis of half porphyrazine iron complex
[0030] Add 0.40 g (0.91 mmol) of the semiporphyrazine ligand and 8.0 mL of N,N-dimethylformamide into a dry round-bottomed flask, stir at room temperature for 0.5 h, and then add 0.24 g (1.20 mmol) FeCl 2 4H 2 O, the reaction was stirred at 155°C for 3 h, cooled to room temperature, and a solid precipitate formed. The reaction solution was filtered under reduced pressure, and the resulting solid was washed with N,N-dimethylformamide, distilled water, absolute ethanol, acetone, and ether to obtain a crude product, which was purified with ethanol and dried in vacuo to obtain 0.37 g of a gray-black product (Fehp), yield 82.0%, melting point >300°C.
[0031] UV-vis (DMSO) λ max : 387 nm.
[0032] IR: 3018 (s, υ =C-H ), 1663, 1572, 1540 (s, υ C=C , υ C=N ), 1467, 1240, 1202 (s, υ C-N , υ C-C ), 765, 730 (s, δ =C-H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com