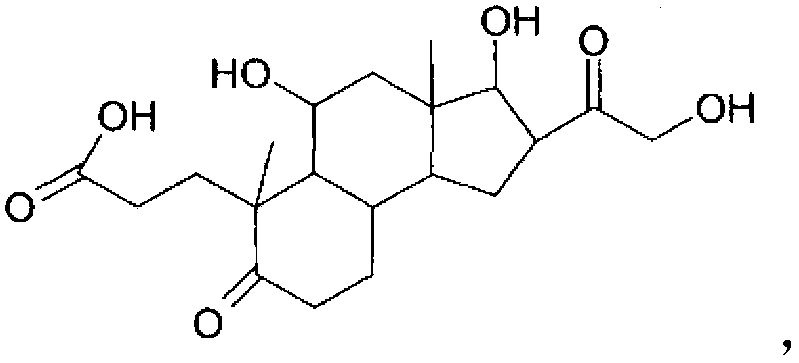
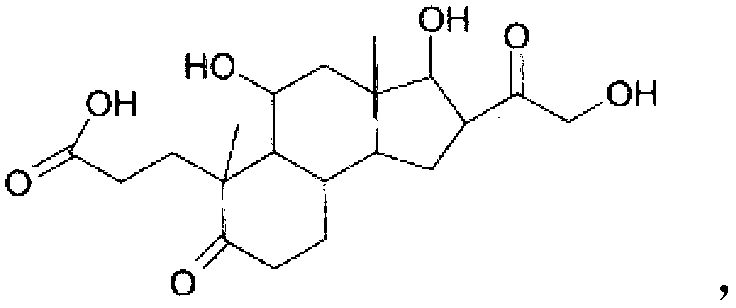
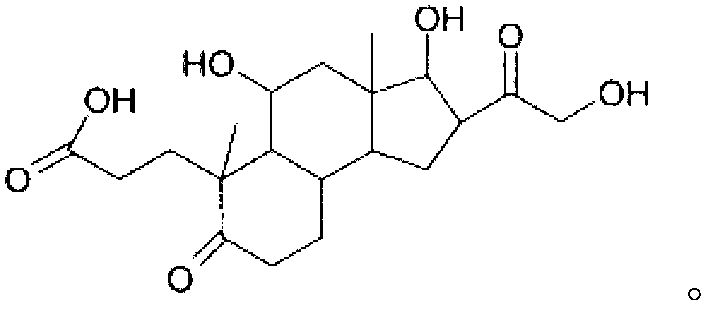
Pharmaceutical compositions and therapeutic applications of a hydrocortisone derivative designated as deina
A compound and composition technology, applied in the field of hydrocortisone derivatives, can solve the problems of tissue atrophy of pine derivatives and the unknown application of complications, and achieve the effect of restoring physiological tissue function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Biopsy and Prototype Protocols
[0160] biopsy
[0161] Animal samples biopsied in the study included biopsies of the following:
[0162] - chronic atrophic skin lesions (basal and marginal) due to venous stasis,
[0163] - damage to articular cartilage (chronic degenerative osteoarthritis),
[0164] - Advanced cicatricial scalp (post-traumatic or surgical injury)
[0165] - Hyperkeratosis of the mouth (vitamin B deficiency).
[0166] Disinfect all samples within 10 minutes with normal saline and antibiotics (penicillin 100 units / ml + streptomycin 100 μg / ml + gentamicin 160 mg / L, fluconazole 0.2 mg / ml) at ambient temperature Wash three times.
[0167] The biopsies were then cut into triplicates (two controls to be processed, 1 and 2, and one sample, 3, for each patient) and suspended on 10 cm slides (Lab-Tek chamber slides, Nunc, Kamstrup, Denmark) in the corresponding culture solution within a final volume of 25 ml.
[0168] Two types of controls were prepar...
Embodiment 2
[0265] Cream Form
[0266] In vivo clinical trials 1.
[0267] conduct clinical trials to evaluate the cream Tolerability and efficacy of the products (compositions shown in Table 5) in regeneration and repair of skin and subcutaneous tissue.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com