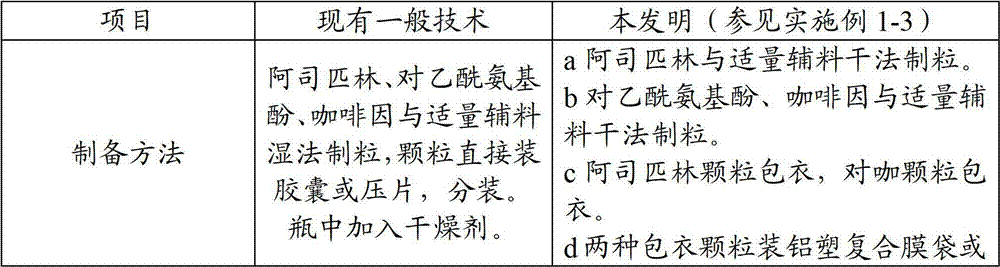
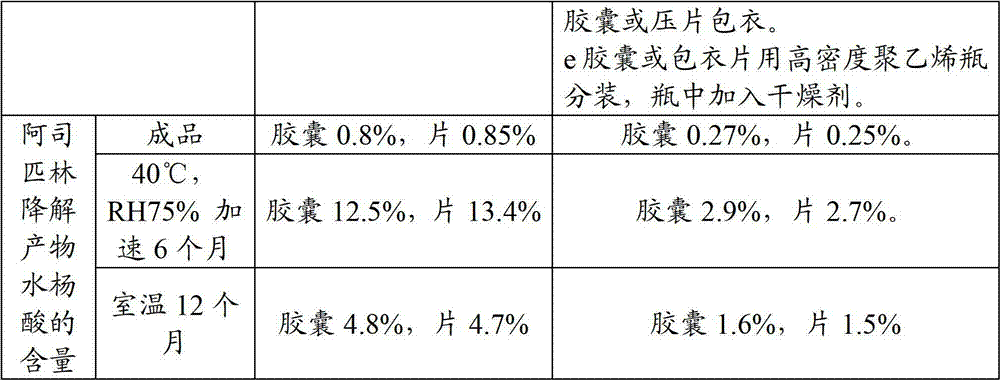
Medicine composition containing aspirin, acetaminophen and caffeine and preparation method thereof
A technology of acetaminophen and aspirin, applied in the field of medicine, can solve the problems of salicylic acid gastrointestinal irritation, unfavorable use of patients, and limitation of clinical application, and achieve the effect of avoiding accelerated degradation and inhibiting degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The pharmaceutical composition containing aspirin, acetaminophen and caffeine described in this embodiment is a granule. The specific formula is as follows:
[0033] prescription:
[0034]
[0035]
[0036] Prescription for granule dispensing
[0037] Aspirin coated granules converted according to content
[0038] The coffee coated granules are converted according to the content
[0039] Preparation:
[0040] a. Aspirin, acetaminophen, caffeine, microcrystalline cellulose, and crospovidone are crushed and passed through an 80-mesh sieve;
[0041] B, aspirin, microcrystalline cellulose, crospovidone and silicon dioxide were weighed according to the prescription amount, then mixed for 30 minutes to obtain mixture 1, and aspirin granules were obtained by dry granulation according to conventional methods;
[0042] c, acetaminophen, caffeine, microcrystalline cellulose, crospovidone and silicon dioxide were weighed according to the prescription amount, and then mi...
Embodiment 2
[0047] The pharmaceutical composition containing aspirin, acetaminophen and caffeine described in this embodiment is a capsule. The specific formula is as follows:
[0048] prescription:
[0049]
[0050]
[0051] Capsule filling prescription
[0052] Aspirin coated granules converted according to content
[0053] Coffee coated granules converted according to content
[0054] Preparation:
[0055] a. Aspirin, acetaminophen, caffeine, microcrystalline cellulose, and crospovidone are crushed and passed through an 80-mesh sieve;
[0056] B, aspirin, microcrystalline cellulose, crospovidone and silicon dioxide were weighed according to the prescription amount, then mixed for 30 minutes to obtain mixture 1; according to conventional methods, dry granulation was used to obtain aspirin granules;
[0057] c, acetaminophen, caffeine, microcrystalline cellulose, crospovidone and silicon dioxide were weighed according to the prescription amount, and then mixed for 30 minutes t...
Embodiment 3
[0062] The pharmaceutical composition containing aspirin, acetaminophen and caffeine described in this embodiment is a tablet. The specific formula is as follows:
[0063] prescription:
[0064]
[0065]
[0066]
[0067]
[0068]
[0069] Preparation:
[0070] a. Aspirin, acetaminophen, caffeine, microcrystalline cellulose, and crospovidone are crushed and passed through an 80-mesh sieve;
[0071] B, aspirin, microcrystalline cellulose, crospovidone and silicon dioxide were weighed according to the prescription amount, then mixed for 30 minutes to obtain mixture 1; according to conventional methods, dry granulation was used to obtain aspirin granules;
[0072] c, acetaminophen, caffeine, microcrystalline cellulose, crospovidone and silicon dioxide were weighed according to the prescription amount, and then mixed for 30 minutes to obtain mixture 2; according to conventional methods, dry granulation made the Coffee particles;
[0073] d. According to the abov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com