Stabilized pharmaceutical composition
A composition and stable technology, applied in the directions of drug combinations, antipyretics, pharmaceutical formulations, etc., can solve problems such as stabilization or reduction that have not yet been discussed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 / A
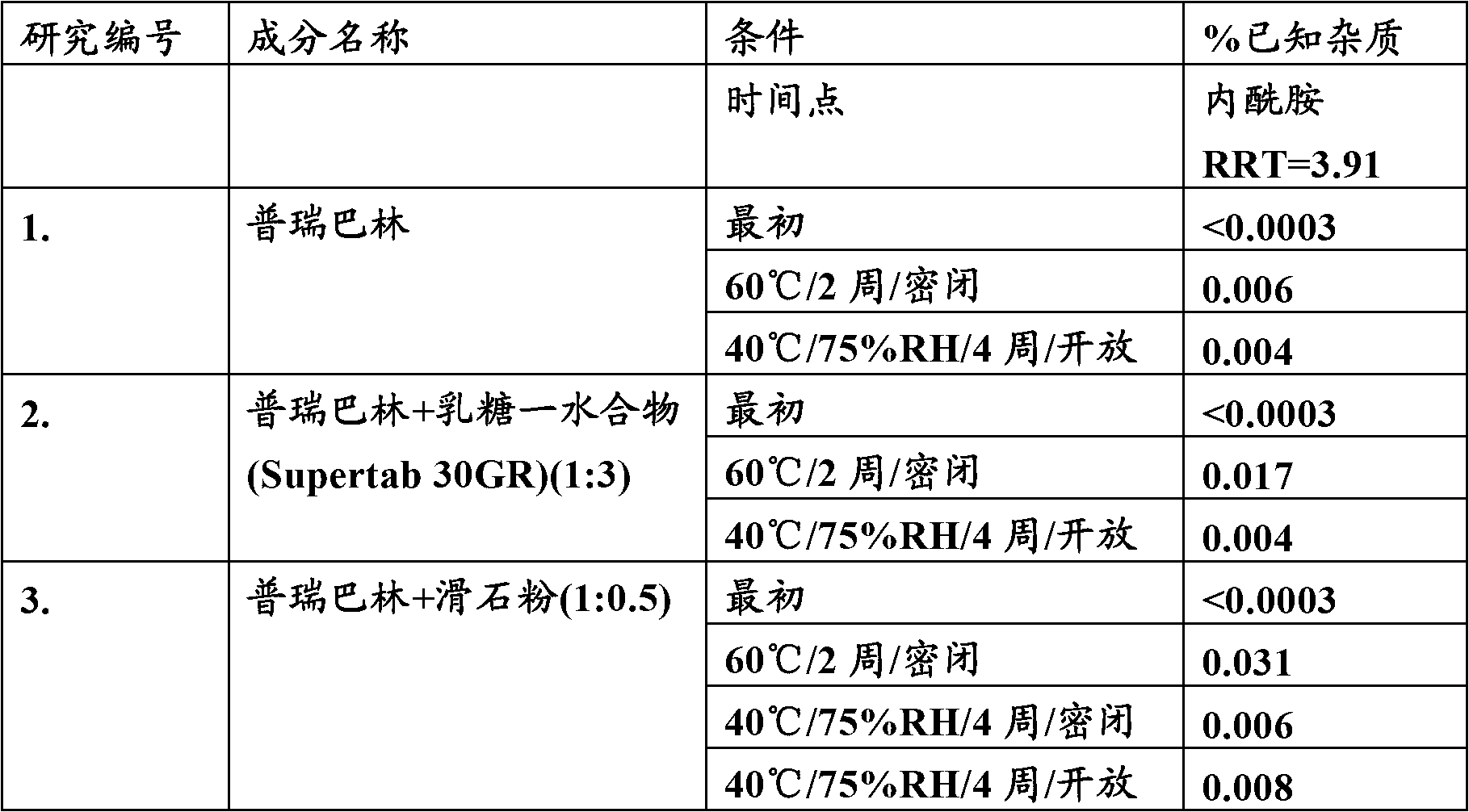
[0034] Comparative evaluation of the stability of pregabalin using different excipients
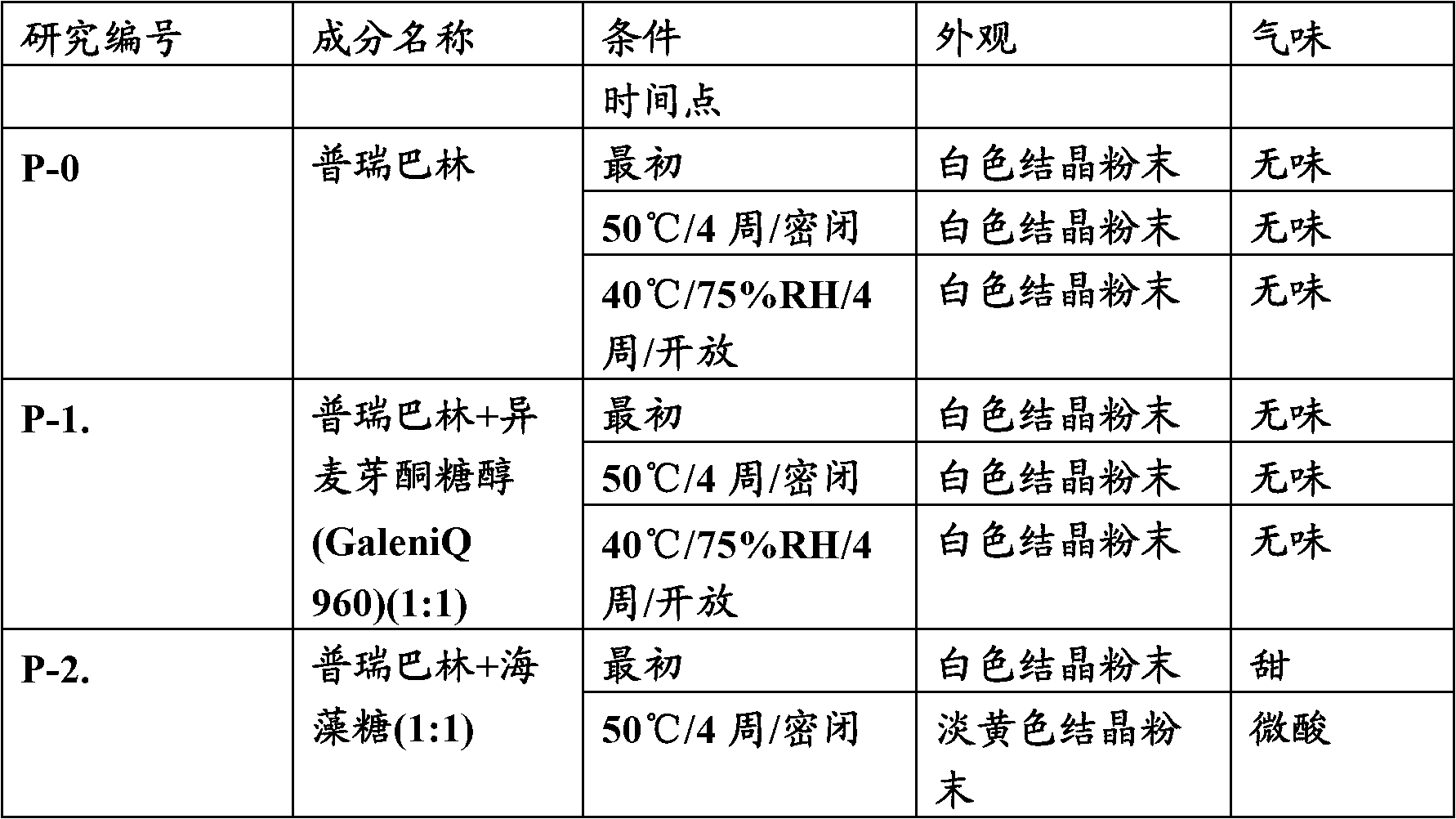
[0035] A preformulation study was performed to evaluate the effect of different physical blends of excipients on the stability of pregabalin. The percentage lactam content in the preformulation mixture was determined by HPLC.
[0036]
[0037]
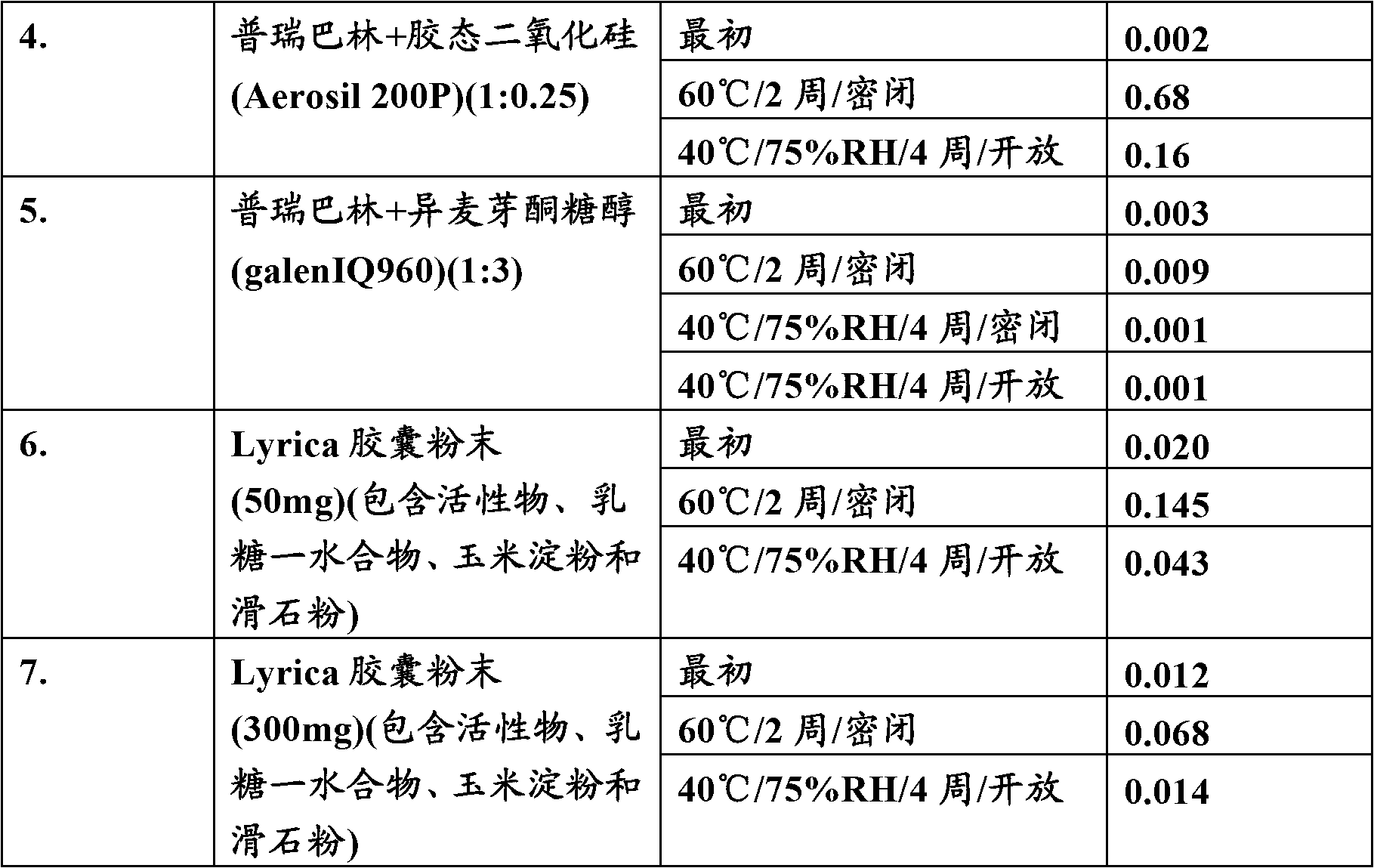
[0038] The above table shows that the degradation of the active pregabalin to the lactam form over time can be prevented by the use of isomalt. It is evident from the above data that, compared with the commercially available formulation In comparison, lactam formation was considerably lower in the preformulation mixture of pregabalin and isomalt.
[0039] Example 1 / B
[0040] Comparative evaluation of the stability of pregabalin using non-reducing sugars
[0041] A preformulation study was performed to evaluate the effect of different physically mixed non-reducing sugars on the stability of pregabalin.
[0042] ...
Embodiment 2
[0045] Pregabalin capsule formulation with 25% drug content (1473-050-25)
[0046] Element
mg / capsule
25
35
Partially Pregelatinized Starch, USP
35
5
total
100
[0047] Pregabalin and all non-talc excipients were sieved and blended. To this blend was added sieved talc and blended further. This drug-excipient blend was filled into capsules using a manual capsule filling machine.
[0048] In Vitro Dissolution Studies
[0049] Use USP II type instrument in 900ml 0.1N HCl with 50rpm to the pregabalin capsule of present embodiment and commercially available Capsules were subjected to in vitro dissolution studies. The comparative dissolution characteristics are made into the following table:
[0050]
[0051] determination
[0052] The pregabalin capsule of the present embodiment is measured and compared with commercially available Preparation compari...
Embodiment 3
[0059] Pregabalin capsule formulation with 25% drug content (1573-093-25)
[0060] Element
mg / capsule
Pregabalin
25
51
Cornstarch, USP
5
19
total
100
[0061] In Vitro Dissolution Studies
[0062] Use USP II type instrument in 900ml 10.1N HCl with 50rpm to the pregabalin capsule of present embodiment and commercially available Capsules were subjected to in vitro dissolution studies. The comparative dissolution characteristics are made into the following table:
[0063]
[0064] determination
[0065] The pregabalin capsule of the present embodiment is measured and compared with commercially available Preparation comparison, the results are as follows:
[0066] preparation
initially
1M 40℃ / 75%RH
LYRICA 25mg
102.9
99.2
Pregabalin capsule of the present invention
102.6
103.7
[0067] Stability study...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com