Method for preparing propylene from ethanol
A technology of ethanol and propylene, which is applied in the field of preparing propylene from ethanol, can solve the problem of low selectivity of propylene, and achieve the effects of increased selectivity, improved reactivity, and good technical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
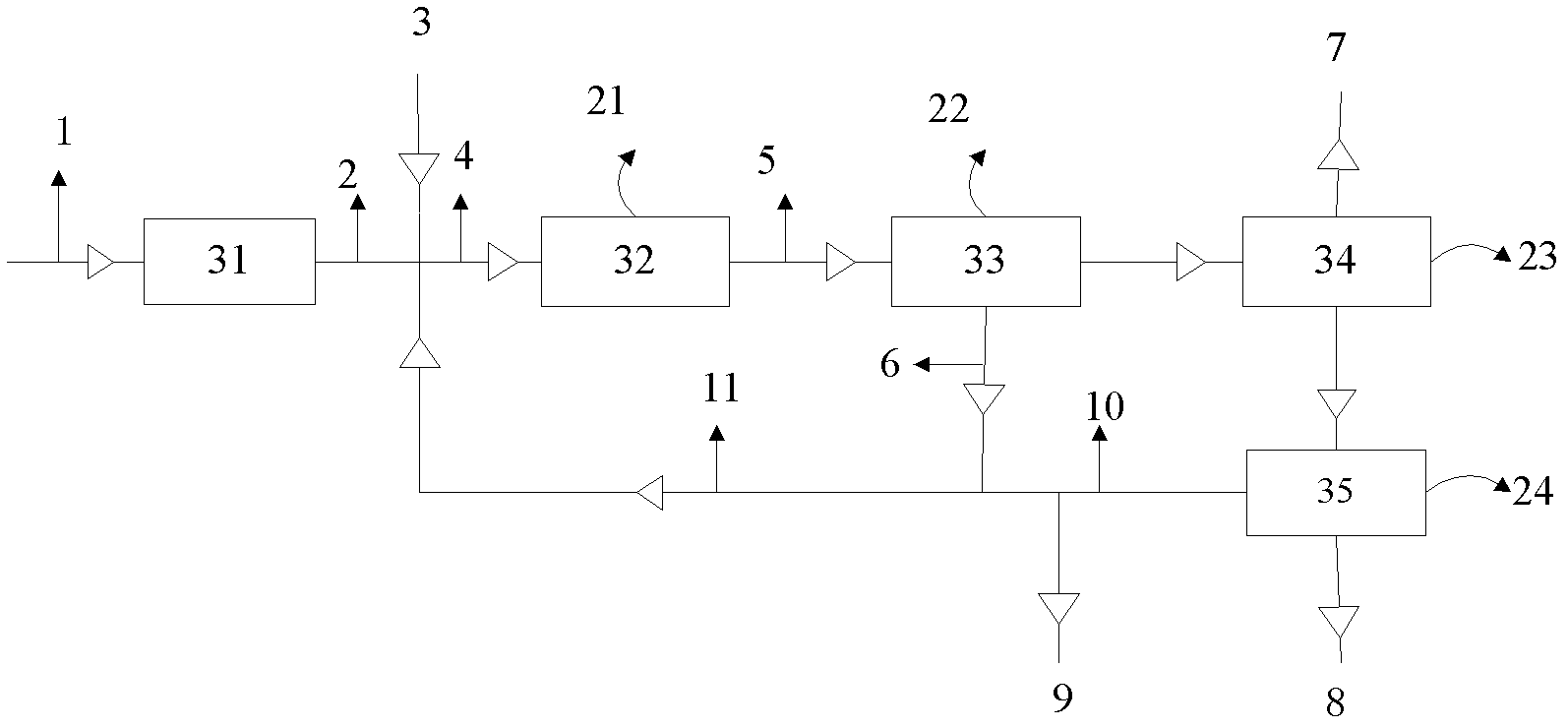
[0019] Send 95% (weight content) of ethanol material 1 to ethanol dehydration reactor 31, and the ethanol in γ-Al 2 O 3 Under the action of the catalyst, the reaction mass space velocity is 0.5 hours at 420℃ -1 The reaction pressure is 0.05MPa to obtain ethylene-containing material 2 with 98% ethylene weight content. The ethylene-containing material 2 is mixed with fresh ether and carbon four 3 to form material 4, and the mixed material 4 enters the reactor 32 for disproportionation reaction , Ethylene and ether after carbon four in 8%WO 3 / SiO 2 Under the action of magnesium oxide, at 300℃, the reaction pressure is 0.5MPa, the mass space velocity of carbon four is 6 hours -1 Under the conditions, the propylene-containing material 5 is obtained, and the material 5 is passed through the deethylene tower 33 and the depropylene tower 34 in order to fractionate unreacted ethylene and the reaction product propylene 7, wherein at least part of the ethylene 6 is recycled to be mixed with...
Embodiment 2
[0021] Send 95% (weight content) of ethanol material 1 to ethanol dehydration reactor 31, and the ethanol in γ-Al 2 O 3 Under the action of the catalyst, the reaction mass space velocity is 0.7 hours at 450℃ -1 The reaction pressure is 0.02MPa to obtain an ethylene-containing material 2 with an ethylene weight content of 97%. The ethylene-containing material 2 is mixed with fresh ether and carbon four 3 to form a material 4, and the mixed material 4 enters the reactor 32 for disproportionation reaction , Ethylene and ether after carbon four in 8%WO 3 / SiO 2 Under the action of magnesium oxide, at 320℃, the reaction pressure is 0.3MPa, the mass space velocity of carbon four is 4 hours -1 Under the conditions, the propylene-containing material 5 is obtained, and the material 5 is passed through the deethylene tower 33 and the depropylene tower 34 in order to fractionate unreacted ethylene and the reaction product propylene 7, wherein at least part of the ethylene 6 is recycled to be...
Embodiment 3
[0023] Send 95% (weight content) of ethanol material 1 to ethanol dehydration reactor 31, and the ethanol in γ-Al 2 O 3 Under the action of the catalyst, the reaction mass space velocity is 0.2 hours at 470℃ -1 The reaction pressure is 0.8MPa to obtain ethylene-containing material 2 with an ethylene content of 97.5% by weight. The ethylene-containing material 2 is mixed with fresh ether and carbon four 3 to form material 4, and the mixed material 4 enters the reactor 32 for disproportionation reaction , Ethylene and ether after carbon four in 8%WO 3 / SiO 2 Under the action of magnesium oxide, at 275℃, the reaction pressure is 0.1MPa, the mass space velocity of carbon four is 8 hours -1 Under the conditions, the propylene-containing material 5 is obtained, and the material 5 is passed through the deethylene tower 33 and the depropylene tower 34 in order to fractionate unreacted ethylene and the reaction product propylene 7, wherein at least part of the ethylene 6 is recycled to be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com