Method for preparing 2, 3, 4-trimethoxybenzaldehyde
A technology of trimethoxybenzaldehyde and trimethoxybenzene, applied in the field of medicine and chemical industry, can solve the problems of unfavorable industrialized production, high price and high production cost, and achieve the effects of low market price, low cost, good yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
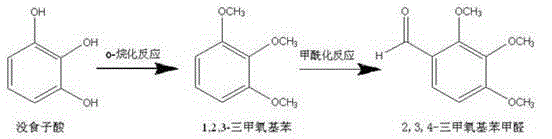
[0019] In a three-neck flask equipped with a thermometer, stirred and cooled by a water bath, add 100 g of raw materials pyrogallic acid and 200 g of water, stir until dissolved, add 330 g of dimethyl sulfate and 400 g of 30% sodium hydroxide dropwise at room temperature, and after the addition is complete, the reactor Keep the temperature at 30°C for 30 minutes. Stand to separate and separate, and wash the upper organic layer with water until neutral to obtain 120g of intermediate 1,2,3-trimethoxybenzene with a yield of 90%
[0020] Add 120g of the above-mentioned intermediate 1,2,3-trimethoxybenzene and 120g of DMF to a three-neck flask equipped with a thermometer, stirring and heating jacket, add 240g of phosphorus oxychloride dropwise, and raise the temperature to 70-80°C for 10 hour, cooled and poured the reaction solution into 500g ice water for hydrolysis, extracted three times with 300ml ethyl acetate, recovered ethyl acetate under reduced pressure, collected the disti...
Embodiment 2
[0022] In a three-neck flask equipped with a thermometer, stirred and cooled by a water bath, add 100 g of raw materials pyrogallic acid and 200 g of water, stir until dissolved, add 365 g of dimethyl sulfate and 400 g of 30% sodium hydroxide dropwise at room temperature, and after the addition is complete, the reactor Keep the temperature at 45°C for 30 minutes. After static separation, the upper organic layer was washed with water to neutrality to obtain 123g of intermediate 1,2,3-trimethoxybenzene with a yield of 92.5%
[0023] Add 123g of the above-mentioned intermediate 1,2,3-trimethoxybenzene and 123g of DMF to a three-neck flask with a thermometer, stirring and heating jacket, add 246g of phosphorus oxychloride dropwise, and heat up to 70-80°C for 10 hour, cooling and pouring the reaction solution into 500g ice water for hydrolysis, extracting three times with 300ml ethyl acetate, recovering ethyl acetate under reduced pressure, collecting the distillate by distillation...
Embodiment 3
[0025] In a three-neck flask equipped with a thermometer, stirred and cooled by a water bath, add 100 g of raw materials pyrogallic acid and 200 g of water, stir until dissolved, add 400 g of dimethyl sulfate and 400 g of 30% sodium hydroxide dropwise at room temperature, and after the addition is complete, the reactor Keep the temperature at 60°C for 30 minutes. After static separation, the upper organic layer was washed with water to neutrality to obtain 123g of intermediate 1,2,3-trimethoxybenzene with a yield of 92.5%
[0026] Add 123g of the above-mentioned intermediate 1,2,3-trimethoxybenzene and 123g of DMF to a three-neck flask with a thermometer, stirring and heating jacket, add 246g of phosphorus oxychloride dropwise, and heat up to 70-80°C for 10 hour, cooling and pouring the reaction solution into 500g ice water for hydrolysis, extracting three times with 300ml ethyl acetate, reclaiming ethyl acetate under reduced pressure, collecting the distillate by distillation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com