Colloidal gold immunofiltration quantitive detection method and reagent kit
A quantitative detection method and detection kit technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of large variation of detection results, time-consuming and laborious, low efficiency, etc., to achieve quality level improvement, cost reduction, and quality requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 prepares the colloidal gold immunofiltration kit that detects CRP
[0028] (1) Preparation of reaction plate
[0029] a. Assemble the absorbent pad, nitrocellulose membrane and plastic shell into a blank reaction plate. There is a round hole in the center of the plastic case.
[0030] b. Coating: spray quality control line 1, detection line, and quality control line 2 on the designated position of the nitrocellulose membrane in the round hole of the plastic shell.
[0031] The detection line is coated with CRP antibody (primary antibody), the concentration is 0.1-2.0mg / ml; the quality control line 1 and the quality control line are coated with CRP standard samples, the concentration is 0.1-2.0mg / ml;
[0032] c. Drying: Dry at 37 degrees for 2 to 24 hours, put it into an aluminum foil bag after cooling, add a bag of desiccant, and seal it.
[0033] (2) Colloidal gold labeling
[0034] a. Label the CRP monoclonal antibody (secondary antibody) to be labele...
Embodiment 2
[0039] The kit obtained in Example 1 was used for detection.
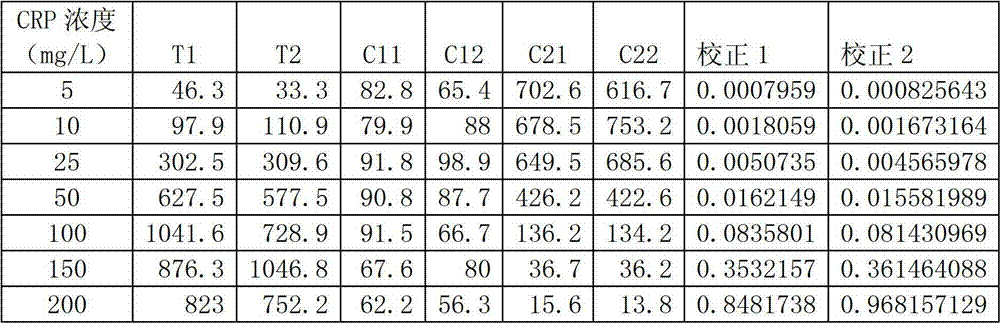
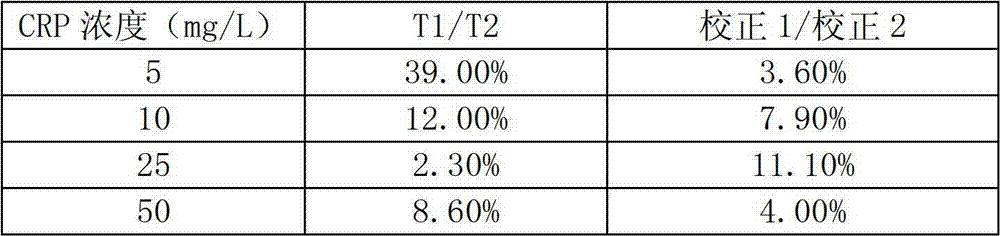
[0040] Take different concentrations of CRP standard sample solutions for testing. The method is to mix 3 μl of different concentrations of CRP standard sample solutions (5, 10, 25, 50, 100, 150 and 200 mg / L) with 200 μl of gold standard solution, Take 120 μl of the mixed solution and add it dropwise on the nitrocellulose membrane in the round hole of the test paper. After it has penetrated completely, add the washing solution and the blocking solution successively.
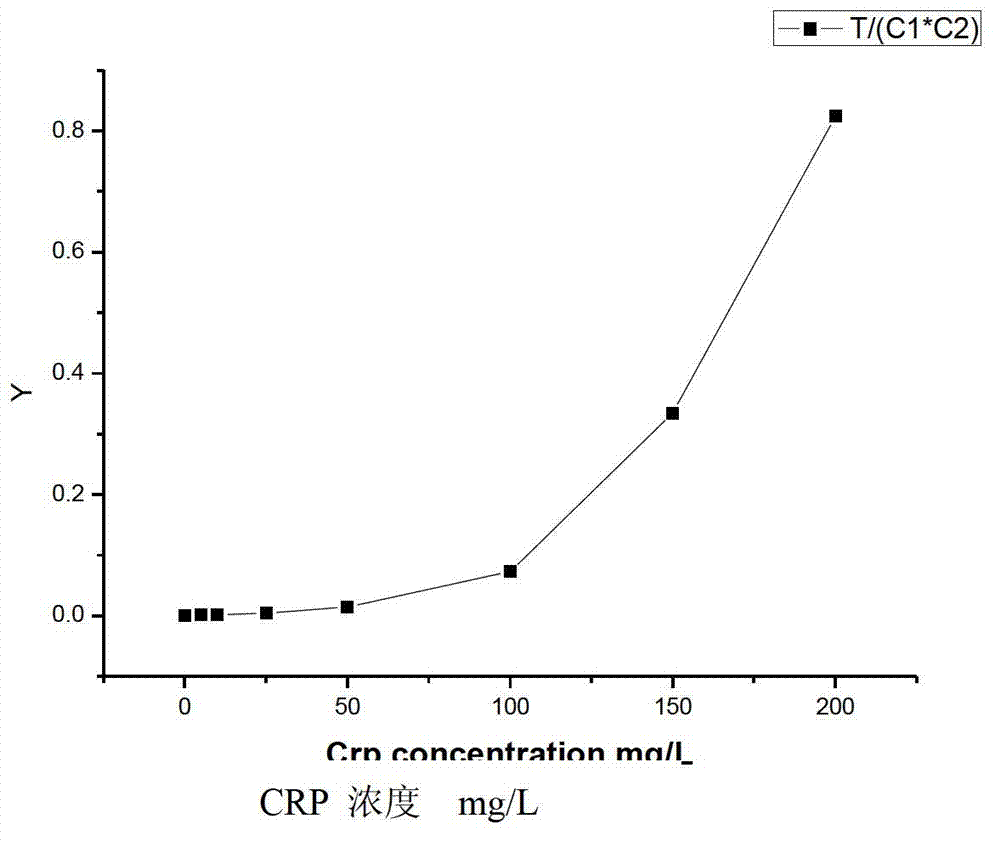
[0041] Put the kit into the quantitative reading instrument, and read the CCD gray value of the test line, quality control line 1 and quality control line 2 at the same time. Calculate with the following formula:
[0042] R=T / (C1*C2), or R=T / C
[0043] Among them, T, C1 and C2 are the internal readings of the test line, quality control line 1 and quality control line 2 respectively (in the case of only one quality control line, the internal reading ...
Embodiment 3
[0058] Embodiment 3 detects the colloidal gold immunofiltration kit of ALB
[0059] The colloidal gold immunofiltration kit for detecting ALB was prepared by the same method as in Example 1, and the CRP and monoclonal antibody were replaced with ALB protein and corresponding monoclonal antibody, and the number of quality control points or lines was one.
[0060] Detect with the method identical with embodiment 2, the result is as table 3:
[0061] table 3
[0062] ALB concentration
T1
T2
C1
C2
T1 / C1
T2 / C2
10mg / L
58.2
70.4
100.3
130.4
0.58
0.54
30mg / L
169.1
138.8
104.4
103.6
1.62
1.34
50mg / L
330
279.6
115.4
100.9
2.86
2.77
100mg / L
485.8
455.8
98.1
89.5
4.95
5.09
200mg / L
706.8
671.2
82.4
83.2
8.58
8.07
[0063] T1------...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com