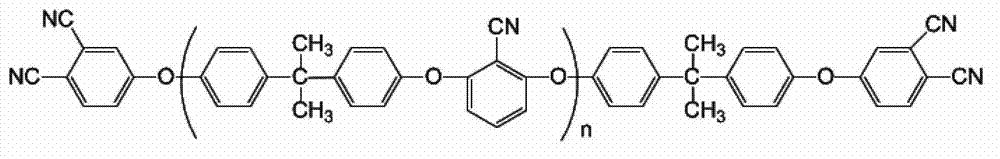
Bisphenol A bisphthalonitrile resin containing arylethernitrile chain segment, cured resin and preparation method thereof
A technology of bisphthalonitrile and aryl ether nitrile, which is applied in the field of bisphenol A bisphthalonitrile resin, cured product and its preparation, can solve the problem of low melting point, post-curing temperature preparation method, and post-curing temperature. Problems such as high curing temperature have not been well resolved to achieve excellent curing molding performance, lower curing processing temperature, and wide processing temperature window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Preparation of bisphenol A type bisphthalonitrile resin containing aryl ether nitrile chain segment (n=2) and its cured product
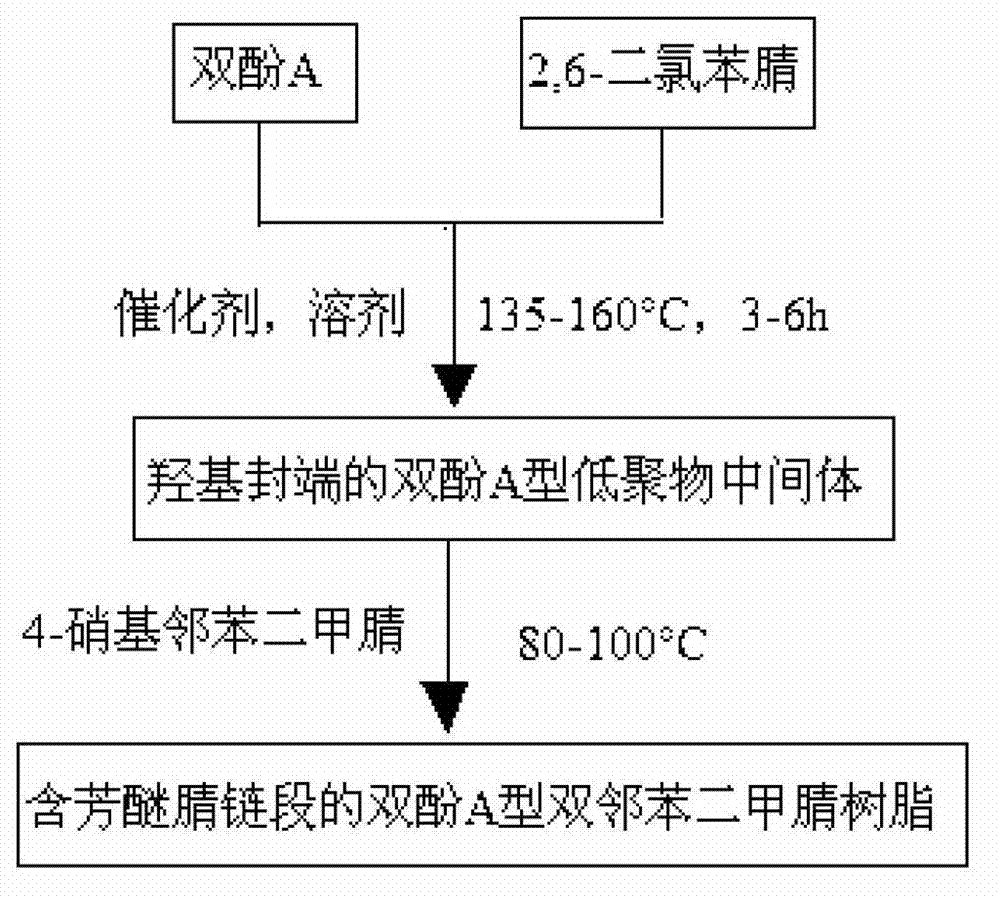
[0062] (1) Synthesis of bisphenol A type bisphthalonitrile resin containing aryl ether nitrile segment (n=2):
[0063] Step 1: Add bisphenol A, 2,6-dichlorobenzonitrile, anhydrous potassium carbonate, N,N-dimethylformamide / toluene mixed solvent into a reaction vessel with water separation device, heat to 130 °C reflux reaction for 6 hours; then emit the by-product water and toluene generated by the reaction in the water separator, continue to heat up and distill out the residual toluene in the reaction system and then cool to room temperature.
[0064] Step 2: Add 4-nitrobisphthalonitrile to the reaction vessel with water separator described in step 1, and react at 90° C. for 6 hours.
[0065] Step 3: the reaction mixture of step 2 is poured into the HCl solution precipitation of 0.1mol / L, filter, wash to neutrality with a large amount of di...
Embodiment 2
[0075] The invention relates to the preparation of the bisphenol A type bisphthalonitrile resin containing aryl ether nitrile chain segment (n=4) and its cured product.
[0076] (1) Synthesis of bisphenol A type bisphthalonitrile resin containing aryl ether nitrile segment (n=4):
[0077] Step 1: Add bisphenol A, 2,6-dichlorobenzonitrile, anhydrous potassium carbonate, N,N-dimethylformamide / toluene mixed solvent into a reaction vessel with water separation device, heat to 150 Reflux reaction under ℃ for 3.5 hours; Then emit the by-product water and toluene generated by the reaction in the water separator, continue to heat up and distill out the residual toluene in the reaction system and then cool to room temperature.
[0078] Step 2: Add 4-nitrobisphthalonitrile to the reaction vessel with a water separator described in step 1, and react at 85° C. for 9.5 hours.
[0079] Step 3: the reaction mixture of step 2 is poured into the HCl solution of 0.1mol / L to precipitate, filter...
Embodiment 3
[0088] The invention relates to the preparation of the bisphenol A type bisphthalonitrile resin containing aryl ether nitrile chain segment (n=6) and its cured product.
[0089] (1) Synthesis of bisphenol A type bis-phthalonitrile resin containing aryl ether nitrile segment (n=6):
[0090] Step 1: Add bisphenol A, 2,6-dichlorobenzonitrile, anhydrous potassium carbonate, and N-methylpyrrolidone / toluene mixed solvent into a reaction vessel with a water separation device, and heat to 160°C for reflux reaction 3 hours; then discharge the by-product water and toluene generated by the reaction in the water separator, continue to heat up and distill out the residual toluene in the reaction system and then cool to room temperature.
[0091] Step 2: Put 4-nitrobisphthalonitrile into the reaction vessel with a water separator described in step 1, and react at 90° C. for 9 hours.
[0092] Step 3: the reaction mixture of step 2 is poured into the HCl solution precipitation of 0.1mol / L, f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com