Smelting method of high-purity and high-nitrogen duplex stainless steel
A technology of duplex stainless steel and smelting method, applied in the field of stainless steel smelting, can solve the problem of high production cost, and achieve the effects of low production cost, good effect and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
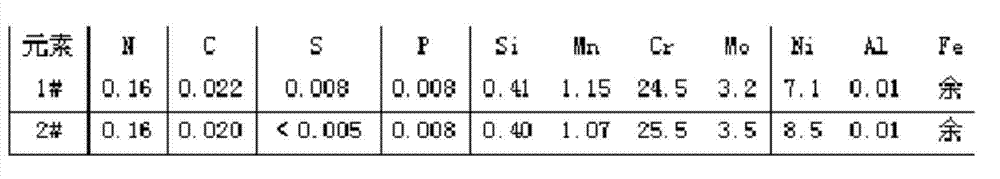
[0019] Example 1: Comparison of the effects of smelting 2507Mo3N duplex stainless steel by using ferrochromium nitride nitrogen-increasing method and this patented method
[0020] Graphite carbon, pure chromium, pure molybdenum, pure nickel, pure iron, aluminum, silicon, manganese and ferrochromium nitride are used for raw material ratio, and ferrochromium nitride is used for smelting: pure iron, pure chromium, pure Add nickel, pure molybdenum, silicon and pot carbon into the crucible of the vacuum induction furnace. After vacuuming to 35Pa, adjust the power of 50kw to heat until the furnace material is completely melted and then smelt for 12 minutes, and then put the pure aluminum material in the bucket of the induction furnace. After deoxidation, it is filled with argon gas for protection, and then put into the bucket of pure manganese, bucket carbon and ferrochromium nitride to deoxidize and adjust the nitrogen content in the steel. After power failure and temperature adjus...
Embodiment 2
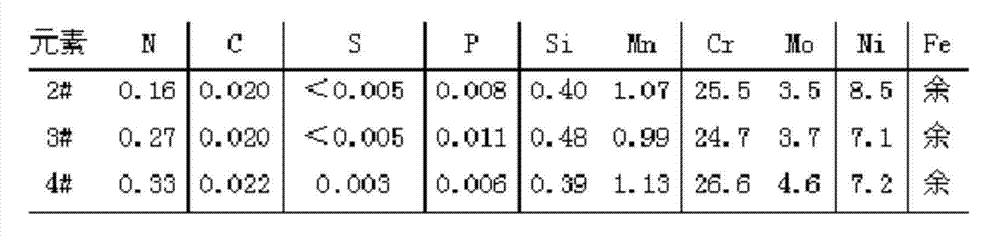
[0026] Example 2: Smelting high-nitrogen duplex stainless steel with different components by using the patented method:
[0027] Graphite carbon, pure chromium, pure molybdenum, pure nickel, pure iron, aluminum, silicon, and manganese are used for raw material ratio to start smelting: add pure iron, pure chromium, pure nickel, pure molybdenum, silicon, and pot carbon into the vacuum induction furnace In the crucible, after evacuating to 35Pa, adjust the power of 50kw to heat until the charge is completely melted and melt for 10 minutes, and then put the pure aluminum material in the bucket of the induction furnace into it. At this time, high-purity nitrogen gas is slowly introduced, and the nitrogen pressure is controlled to be 0.60 MPa, and the power is adjusted to 20 kw for further refining for 12 minutes. After refining, put the pure manganese and bucket carbon into the bucket for deoxidation. After power failure and temperature adjustment, the steel was tapped and smelted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com