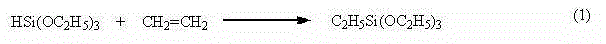Preparation method of ethyltriethoxysilane
A technology of ethyltriethoxysilane and triethoxysilane, which is applied in the field of preparation of intermediate ethyltriethoxysilane, can solve the problems of large investment in reaction equipment, large environmental hazards, and high cost of industrial production. Achieve the effect of low price, good effect and saving operation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 1597.7 g (9.726 mol) of raw material triethoxysilane, catalyst RuCl 3 ·xH 2 0.0201g (8.514×10 -5 mol), cocatalyst CuCl 0.0064 g (6.465×10 -5 mol);
[0028] (2) After stirring, the temperature of the reaction system was raised to 65-75°C and maintained for 32 minutes, and 0.35MPa ethylene gas was introduced into the synthesis kettle from the gas bottom pipe. After the reaction was initiated, the reaction temperature was maintained at 65-75°C and stirred for reaction;
[0029] (3) When the ethylene inlet valve is closed and the pressure in the synthesis kettle no longer decreases, the reaction is stopped, the materials in the kettle are lowered to room temperature and collected, and the samples are analyzed by gas chromatography GC (HP-5 chromatographic column, column temperature 120°C, vaporization chamber 200°C, sampling chamber 200°C, FID detector), the content of ethyltriethoxysilane is 97.29%, and the content of tetraethoxysilane is 2.52%. Calculate th...
Embodiment 2
[0034] (1) Add 382.4 g (2.328 mol) of raw material triethoxysilane, catalyst RuCl 3 ·xH 2 O 0.0387g (1.639×10 -4 mol), cocatalyst CuCl 0.1475 g (1.490×10 -3 mol);
[0035] (2) Heat up the temperature of the reaction system to 75-80°C with stirring and maintain it for 30 minutes, then feed 0.25MPa ethylene gas into the synthesis kettle from the gas bottom pipe, after the reaction is initiated, keep the reaction temperature at 75-85°C and stir for reaction;
[0036] (3) When the ethylene inlet valve is closed and the pressure in the synthesis kettle no longer decreases, the reaction is stopped, the materials in the kettle are lowered to room temperature and collected, and the samples are analyzed by gas chromatography GC (HP-5 chromatographic column, column temperature 120°C, vaporization chamber 200°C, sampling chamber 200°C, FID detector), the content of ethyltriethoxysilane is 97.63%, and the content of tetraethoxysilane is 2.20%. Calculate the content of ethyltriethoxys...
Embodiment 3
[0039] (1) Add 300.4 g (1.829 mol) of raw material triethoxysilane, catalyst RuCl 3 ·xH 2 O 0.0557g (2.359×10 -4 mol), cocatalyst CuCl 0.1454 g (1.469×10 -3 mol);
[0040] (2) After stirring, raise the temperature of the reaction system to 55-65°C and maintain it for 14 minutes, then pass 0.35MPa ethylene gas into the synthesis kettle from the gas bottom pipe, after the reaction is initiated, keep the reaction temperature at 55-65°C and stir the reaction;
[0041] (3) When the ethylene inlet valve is closed and the pressure in the synthesis kettle no longer decreases, the reaction is stopped, the materials in the kettle are lowered to room temperature and collected, and the samples are analyzed by gas chromatography GC (HP-5 chromatographic column, column temperature 120°C, vaporization chamber 200°C, sampling chamber 200°C, FID detector), the content of ethyltriethoxysilane is 97.83%, and the content of tetraethoxysilane is 1.91%. Calculate the content of ethyltriethoxys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com