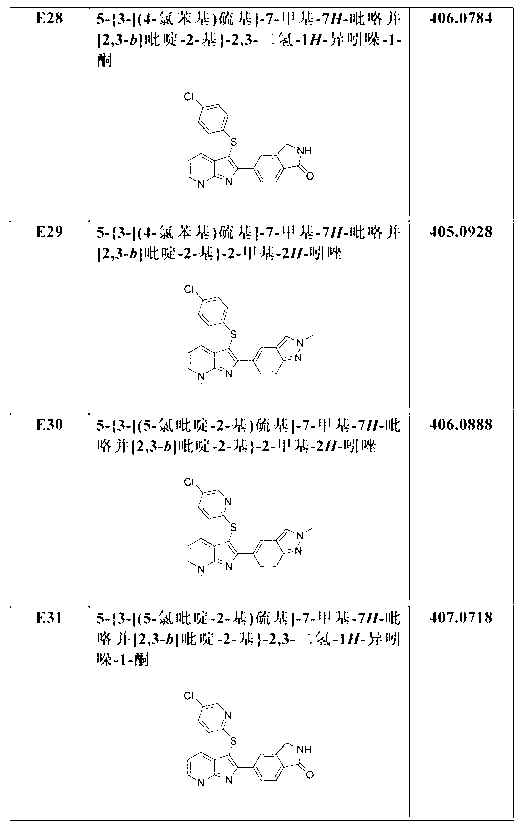
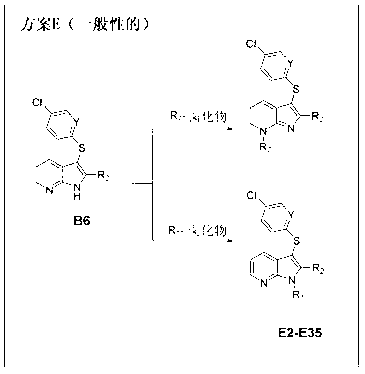
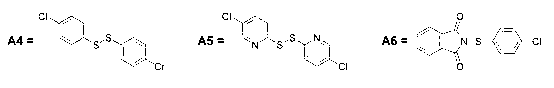Aza-indole derivatives useful as modulators of FAAH
A compound, CH2 technology, applied in the direction of active ingredients of heterocyclic compounds, anti-toxic agents, anti-inflammatory agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0473] Preparation of lysates and microsomes
[0474] CHO cells expressing FAAH were used to prepare crude cell lysates or microsomal fractions. To harvest the cells, decant the tissue culture medium slowly and replace the monolayer with Ca-free ++ Mg ++ The cells were washed three times with PBS and recovered after 15 min in enzyme-free dissociation medium (Millipore Corp, Billerica, MA). Cells were harvested by centrifugation at 2000 rpm for 15 min, and the cell pellet was resuspended with 50 mM HEPES (pH 7.4) containing 1 mM EDTA and the protease inhibitors aprotinin (1 mg / ml) and leupeptin (100 μM). hanging. Cell lysates were recovered after sonicating the suspension at 4°C and centrifuging at 12,000xg (14,600rpm, SS34 rotor) for 20 min at 4°C to form cell debris, nuclei, peroxisomes, lysosomes, and mitochondria crude pellets; supernatants or cell lysates were used for FAAH enzyme assays. In some cases, FAAH-enriched microsomal fractions were prepared by further cen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com