Preparation method of high-purity vanadium pentoxide
A high-purity vanadium pentoxide technology, which is applied in the field of preparation of high-purity vanadium pentoxide by multi-stage remelting operation, can solve the problems of increased dosage, complicated operation, and long process flow, and achieves low environmental pollution , easy operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The invention provides a kind of preparation method of high-purity vanadium pentoxide, and described preparation method comprises the following steps:
[0015] (1) Redissolving the crude vanadium in the alkali solution, and then filtering to remove metal impurities in the crude vanadium that precipitated with the alkali solution to obtain the first-stage return solution.
[0016] Wherein, the crude vanadium is metallurgical grade vanadium pentoxide, and the crude vanadium used in the present invention contains metal ion impurities such as chromium and sodium and non-metal ion impurities such as silicon. Preferably, the alkali solution adopts sodium hydroxide solution, and further, the sodium hydroxide solution can adopt analytically pure sodium hydroxide solution, of course other can dissolve the vanadium in the crude vanadium and react with the metal cation in the crude vanadium to generate hydroxide Alkaline solutions for the precipitation of substances are acceptable...
example 1
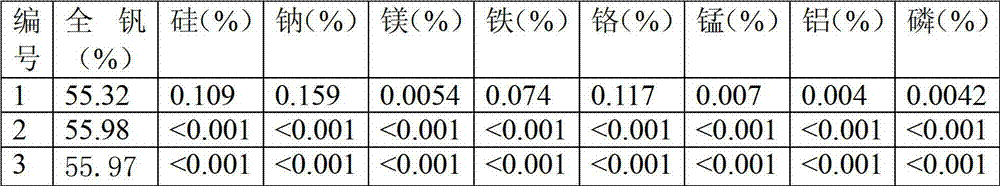
[0023] The thick vanadium of 100g is redissolved in sodium hydroxide solution (made by mixing 50g sodium hydroxide solid and 400ml deionized water), and the pH value of the resulting solution is 8.93, and then filtered to remove insoluble impurities to obtain the first-level return solution; Prepare a sulfuric acid solution with 200ml of concentrated sulfuric acid and 600ml of deionized water, then slowly pour the resulting 1100ml first-stage return solution into the prepared sulfuric acid solution, while stirring, and adjust the pH value to 1.0. The continuous addition of the first-stage return solution produces a large amount of red solid, and the viscosity of the solution increases. At this time, deionized water is added at a liquid-solid ratio of 1:1 to dilute the solution so that impurities Si enter the liquid phase and vanadium enters the solid phase. , to obtain a solid-liquid mixture, and realize solid-liquid separation by suction filtration. After the precipitate obtai...
example 2
[0025] The thick vanadium of 100g is redissolved in sodium hydroxide solution (made by mixing 50g sodium hydroxide solid and 400ml deionized water), and the pH value of the resulting solution is 8.93, and then filtered to remove insoluble impurities to obtain the first-level return solution; Prepare a sulfuric acid solution with 200ml of concentrated sulfuric acid and 600ml of deionized water, then slowly pour the resulting 1100ml first-stage return solution into the prepared sulfuric acid solution, while stirring, and adjust the pH value to 2.0. The continuous addition of the first-stage return solution produces a large amount of red solid, and the viscosity of the solution increases. At this time, deionized water is added at a liquid-solid ratio of 1:1 to dilute the solution so that impurities Si enter the liquid phase and vanadium enters the solid phase. , to obtain a solid-liquid mixture, and realize solid-liquid separation by suction filtration. After the precipitate obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com