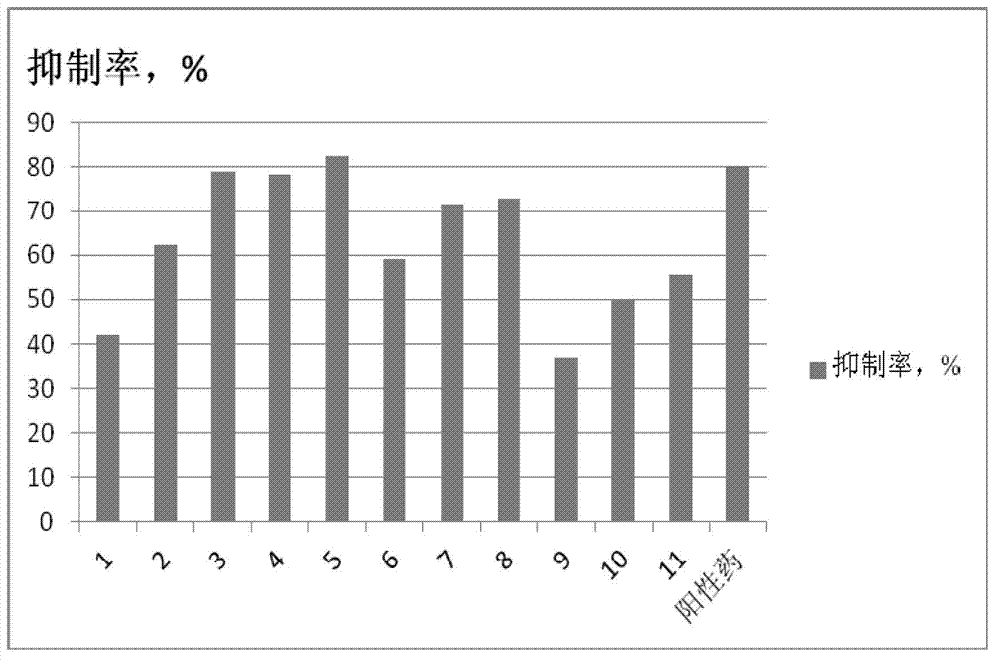
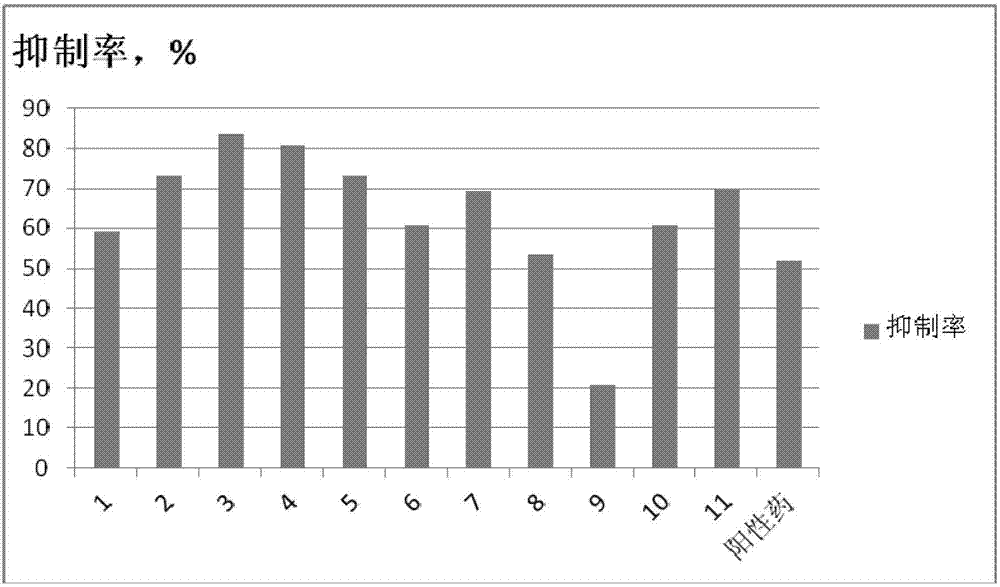
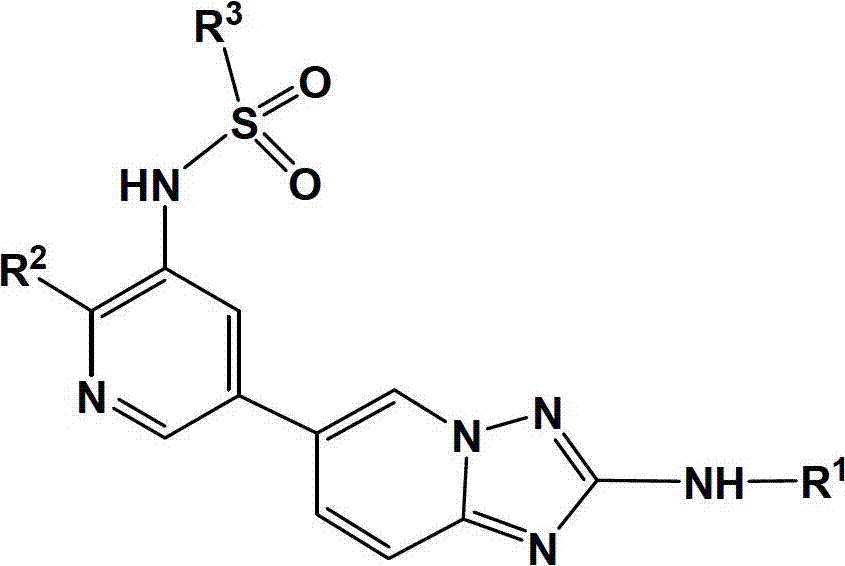
6-(5-pyridyl)-1,2,4-triazolopyridine compound, and preparation method and application thereof
A technology based on azolopyridine and pyridyl, which is applied in the field of anti-tumor compounds, can solve the problems of poor solubility, high production cost, and difficulty in the synthesis of imidazopyridazine derivatives, and achieves the advantages of easy synthesis method and easy-to-obtain synthetic raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 2-Acetamido-6-[2-chloro-3-(4-fluorobenzenesulfonylamino)-5-pyridyl]-1,2,4-triazol[1,5-α]pyridine (1) Preparation
[0048] Intermediate A: Preparation of 2-chloro-3-(4-fluorobenzenesulfonylamino)-5-bromopyridine (1A)
[0049] Reference J. Med. Chem. 2011, 54, 4735–4751 Synthesis.
[0050] Intermediate B: Preparation of 2-acetyl-6-[1,2,4-triazol[1,5-α]pyridinyl]boronic acid pinacol ester (1B).
[0051] Synthesized by reference WO 2009068482A1.
[0052] Synthesis of Compound 1:
[0053] Add 2-chloro-3-(4-fluorobenzenesulfonylamino)-5-bromopyridine (0.19g), 2-acetyl-6-[1,2,4-triazole[1 ,5-α]pyridyl]boronic acid pinacol ester (0.16g), PdCl 2 (pddf) (25mg), sodium carbonate (0.08g), ethylene glycol dimethyl ether (7.0mL), water (2.0mL), ethanol (3.0mL), the mixture was stirred and refluxed for 1h under nitrogen protection, and evaporated under reduced pressure The solvent and the residue were separated by silica gel column chromatography (chloroform: methanol ...
Embodiment 2
[0054] Example 2 2-Acetamido-6-[2-chloro-3-(4-methylbenzenesulfonylamino)-5-pyridyl]-1,2,4-triazol[1,5-α] Preparation of pyridine (2)
[0055] Reference J.Med.Chem.2011, 54, 4735-4751 Synthesis of 2-chloro-3-(4-methylbenzenesulfonylamino)-5-bromopyridine.
[0056] Replace 2-chloro-3-(4-fluorobenzenesulfonylamino)-5-bromopyridine with 2-chloro-3-(4-methylbenzenesulfonylamino)-5-bromopyridine, and the others are the same as in Example 1 Synthesis of compound 1. Yield 57.4%. 1 H-NMR: δ10.89(s,1H,NH),10.39(s,1H,NH),9.34(s,1H,Ar-H),8.68(s,1H,Ar-H),8.10(s, 1H,Ar-H),7.91(d,J=9.2Hz,1H,Ar-H),7.82(d,J=9.2Hz,1H,Ar-H),7.65(d,J=8.0Hz,2H, Ar-H)7.38(d,J=7.6Hz,2H,Ar-H),2.39(s,3H,CH 3 ),2.16(s,3H,CH 3 ).
Embodiment 3
[0057] Example 3 2-Acetamido-6-[2-methoxy-3-(4-fluorobenzenesulfonylamino)-5-pyridyl]-1,2,4-triazol[1,5-α] Preparation of pyridine (3)
[0058] Reference WO 2008157191A2 Synthesis of 2-methoxy-3-(4-fluorobenzenesulfonylamino)-5-bromopyridine.
[0059] Replace 2-chloro-3-(4-fluorobenzenesulfonylamino)-5-bromopyridine with 2-methoxy-3-(4-fluorobenzenesulfonylamino)-5-bromopyridine, and the others are the same as in Example 1 Synthesis of compound 1. Yield 70.4%. 1 H-NMR: δ10.84(s,1H,NH),10.08(s,1H,NH),9.23(s,1H,Ar-H),8.40(s,1H,Ar-H),8.00(s, 1H,Ar-H),7.90(d,J=9.2Hz,1H,Ar-H),7.81(m,3H,Ar-H),7.41(m,2H,Ar-H),3.64(s,3H ,OCH 3 ),2.16(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com