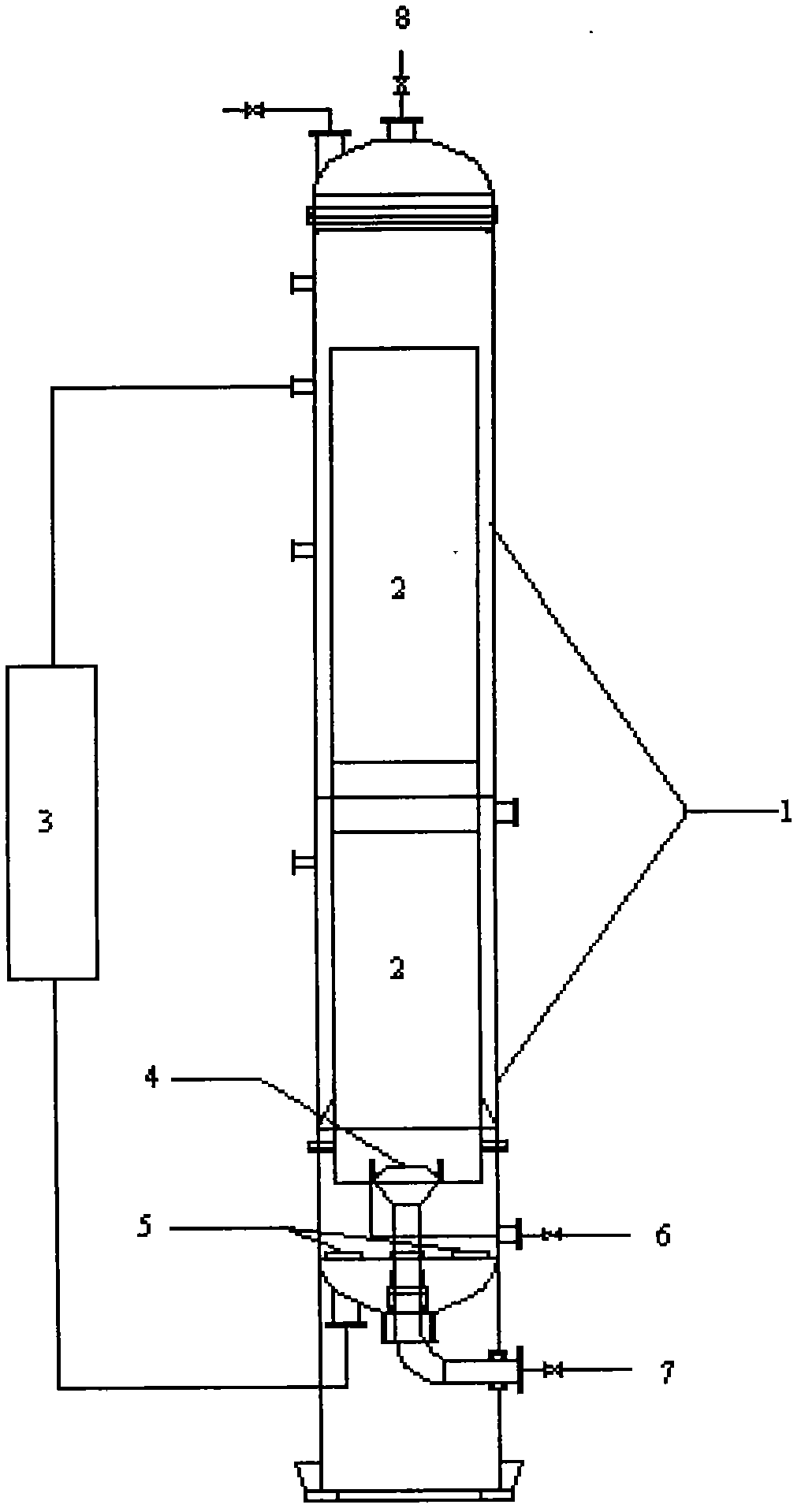
New tower type oxidation technology for indigo blue
A kind of indigo and tower technology, applied in the field of high-efficiency synthesis of indigo, can solve the problems of uneven gas distribution, easy foaming reaction time, large washing loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The ultrasonic generator used in this experiment has a frequency of 25KHz and a power of 100W.
[0018] After adding 200g of alkali melt into 800g of water to dissolve (the temperature is 71.6°C), transfer the material to an airlift reactor, turn on the ultrasonic wave, and feed air into the reactor to keep the temperature of the material in the reactor at 68-72 ℃, reacted for 3 hours, reached the end of the reaction, filtered and washed the reaction material, and obtained the indigo product. In the filtrate, the filter value of indigo is 3.0g / L, the content of carbonate is 13.2g / L, the alkalinity is 15%, the content of indigo after drying is 95.6%, the moisture is 0.40%, and the iron ion is 86ppm.
Embodiment 2
[0020] The ultrasonic generator used in this experiment has a frequency of 25KHz and a power of 100W.
[0021] After adding 200g of alkali melt into 800g of experiment 1's alkali water (alkali concentration is 15%) and dissolving (alkali concentration is 25%, temperature is 75.6°C), the material is transferred to the air-lift reactor, and the ultrasound is turned on. Air is introduced into the reactor, the temperature of the materials in the reactor is kept at 68-72° C., and the pressure is 0.15 Mp. The reaction is carried out for 5 hours until the end of the reaction is reached. After the reaction materials are filtered and washed, the indigo product is obtained. In the filtrate, the filter value of indigo is 5.4g / L, the content of carbonate is 21.3g / L, the alkalinity is 36%, the content of indigo after drying is 95.1%, the moisture is 0.38%, and the iron ion is 114ppm.
Embodiment 3
[0023] The ultrasonic generator used in this experiment has a frequency of 25KHz and a power of 100W.
[0024] After adding 400g of alkali melt into 800g of water for dissolution (the alkali concentration is 25%, and the temperature is 80.6°C), the material is transferred to the air-lift reactor, the ultrasonic is turned on, and air is introduced into the reactor to keep the reactor inside The temperature of the material is 68-72° C., the pressure is 0.15 Mp, and the reaction is carried out for 6 hours until the end of the reaction is reached. After the reaction material is filtered and washed, the indigo product is obtained. In the filtrate, the filter value of indigo is 4.8g / L, the content of carbonate is 18.2g / L, the alkalinity is 38%, the content of indigo after drying is 95.3%, the moisture is 0.42%, and the iron ion is 109ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com