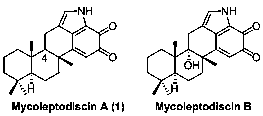
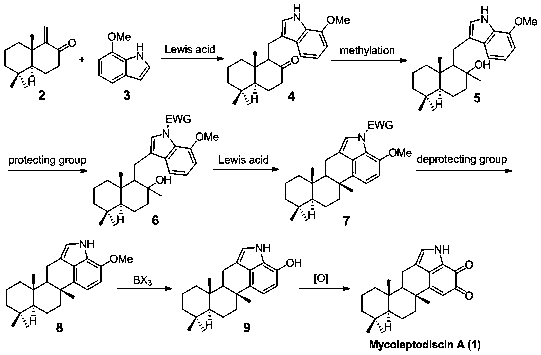
A kind of synthetic method of mycoleptodiscin A
A synthetic method and technology of sesquiterpene, which is applied in the field of synthesis of marine natural product mycoleptodiscinA, can solve the problems of long reaction route, harsh reaction conditions, unsuitability for industrial production, etc., and achieve high overall yield, few reaction steps, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of sesquiterpene indole 4
[0027] Dissolve 3.09 g (2, 15.0 mmol) of enone sesquiterpene and 2.2 g (3, 15.0 mmol) of 7-methoxyindole in 100 ml of 1,2-dichloroethane, add trifluoromethanesulfonic acid 0.63 g (1.5 mmol) of tin was stirred and reacted at room temperature for 3 hours, and the reaction was detected by TLC. Add 100 ml of water, wash the reaction liquid, extract the aqueous phase with dichloromethane (30 mL x 2), wash the combined organic phase with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain 5.09 g of a white solid. The yield was 96%.
[0028] H NMR spectrum data: 1 H-NMR ((400 MHz, CDCl 3 ): δ = 8.14 (br, s, 1 H), 7.20(d, J = 7.9 Hz, 1 H), 7.04 (d, J = 7.7 Hz, 1 H), 7.00 (s, 1 H), 6.63 ( d, J =7.6 Hz, 1 H), 3.94 (s, 3 H), 3.19 (dd, J = 13.9, 10.1 Hz, 1 H), 2.68 (d, J =14.1 Hz, 1 H), 2.49 (d , J = 9.7 Hz, 1 H), 2.38 (dd, J = 12.8, 2.4 Hz, 1 H), 2.21 (td...
Embodiment 2
[0030] Embodiment 2: the synthesis of sesquiterpene indolol 5
[0031] Take 4.24 g of sesquiterpene indole (4, 12.0 mmol) and dissolve it in 100 ml of anhydrous tetrahydrofuran. Repeat argon filling and exhausting three times to exhaust the air. The temperature was lowered to -10 °C, and 11.25 ml (1.6 M, 18 mmol) of methyllithium was slowly added dropwise, and the reaction was stirred at this temperature for 2 hours, and the reaction was detected by TLC. Add 50 ml of saturated sodium carbonate solution to the reaction system, extract with ether (50 mL x 3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain 4.16 g of white solid , the yield is 94%.
[0032] H NMR spectrum data: 1 H-NMR ((400 MHz, CDCl3 ): δ = 8.29 (br, s, 1 H), 7.33(d, J = 7.9 Hz, 1 H), 7.07 (d, J = 7.8 Hz, 1 H), 6.94 (s, 1 H), 6.67 ( d, J =7.6 Hz, 1 H), 3.97 (s, 3 H), 2.97 (dd, J = 16.7, 5.7 Hz, 1 H), ...
Embodiment 3
[0034] Example 3: Synthesis of N-phenylsulfonyl protected sesquiterpene indole 6
[0035] Dissolve 3.69 g (5, 10.0 mmol) of sesquiterpene indole in 100 ml of toluene, cool down to 0 °C, add 0.68 g (2 mmol) of tetrabutylammonium bisulfate, 50 wt% sodium hydroxide in sequence 100 ml of aqueous solution, slowly dropwise added 1.54 ml (12 mmol) of phenylsulfonyl chloride. The temperature was raised to room temperature, and the stirring reaction was continued for 2 hours, and TLC detected that the reaction was complete. Add 50 ml of water to the reaction system, separate the layers, extract the aqueous phase with dichloromethane (50 mL x 3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain 4.89 g of light yellow foamy solid, yield 96%.
[0036] H NMR spectrum data: 1 H-NMR ((400 MHz, CDCl 3 ): δ = 7.80 (d, J = 8.1 Hz, 1H), 7.57 (s, 1 H), 7.52 (m, 1 H), 7.44 (t, J = 7.5 Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com