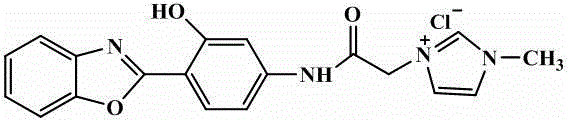
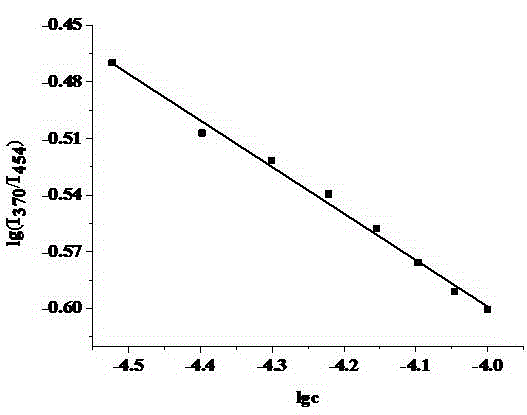
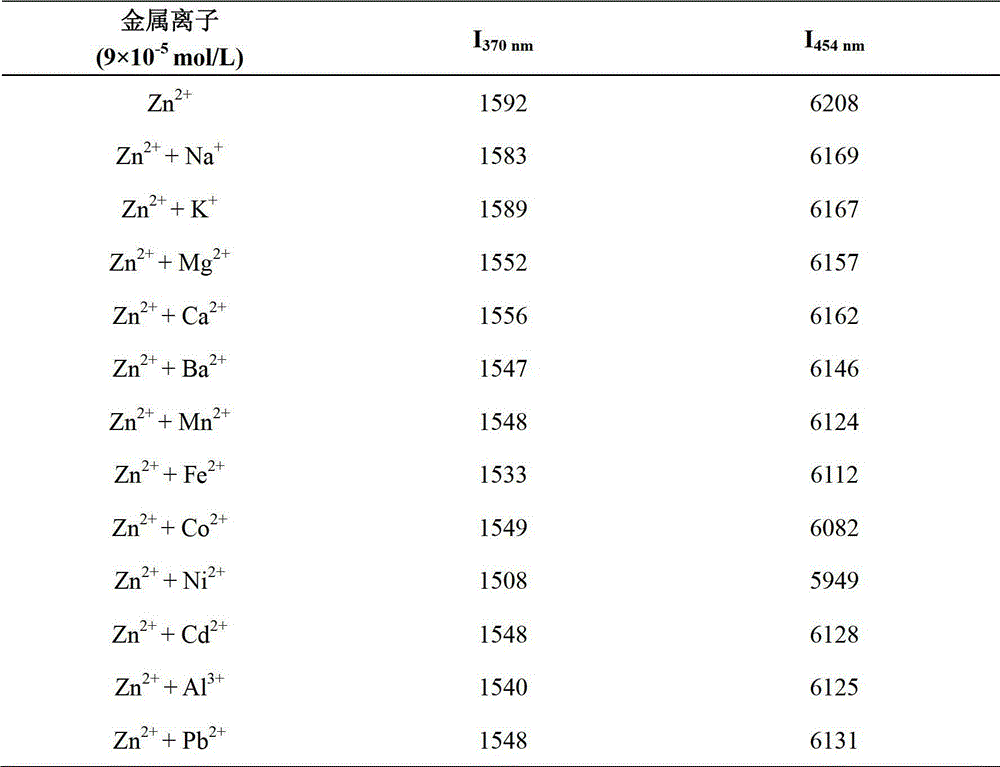
Zn<2+> ratiometric fluorescent probe compound and preparation method and use thereof
A ratiometric fluorescent probe and compound technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve problems such as limited applications, and achieve the effects of increased sensitivity, elimination of system errors, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Add 11.3 g of 2-(4'-amino-2'-hydroxyphenyl)benzoxazole, 100 ml of dichloromethane and 10 ml of 20% NaOH aqueous solution to a 250 mL three-neck flask, and stir at 0 °C Add dropwise 5.7 g of chloroacetyl chloride, and dropwise finishes within 20 minutes;
[0026] (2) Continue to stir at room temperature for 2 hours, let stand to separate layers, take the water layer, and set aside;
[0027] (3) The aqueous layer solution was extracted 3 times with dichloromethane, and the organic layers were combined, and the organic layers were successively washed with 5% HCl and 5% NaHCO 3 aqueous solution and washed with water to neutral, anhydrous MgSO 4 After drying, filtering, and evaporating the solvent under reduced pressure, 13.9 g of the intermediate compound was obtained with a yield of 92%.
[0028] (4) Dissolve 13.9 g of the intermediate compound in 50 mL of acetonitrile, then add 4.5 g of N-methylimidazole, heat to reflux for 24 h, distill off the acetonitrile to obta...
Embodiment 2
[0030] (1) Add 11.3 g of 2-(4'-amino-2'-hydroxyphenyl)benzoxazole, 100 mL of dichloromethane and 10 ml of 20% NaOH aqueous solution into a 250 mL three-neck flask, and drop them under stirring at 0 °C Add 7.4g of chloroacetyl chloride, and dropwise finish within 40 minutes;
[0031] (2) Continue to stir at room temperature for 1 hour, let stand to separate layers, take the water layer, and set aside;
[0032] (3) The aqueous layer solution was extracted 3 times with dichloromethane, and the organic layers were combined, and the organic layers were successively washed with 5% HCl and 5% NaHCO 3 aqueous solution and washed with water to neutral, anhydrous MgSO 4 After drying, filtering, and evaporating the solvent under reduced pressure, 14.2 g of the intermediate compound was obtained with a yield of 94%.
[0033](4) Dissolve 14.2 g of the intermediate compound in 50 mL of acetonitrile, then add 3.9 g of N-methylimidazole, heat to reflux for 18 h, distill off the acetonitrile...
Embodiment 3
[0035] (1) Add 11.3 g of 2-(4'-amino-2'-hydroxyphenyl)benzoxazole, 100 mL of dichloromethane and 10 ml of 20% NaOH aqueous solution into a 250 mL three-necked flask, and drop them under stirring at 5 °C Add 6.3g of chloroacetyl chloride, and the dropwise addition ends within 33 minutes;
[0036] (2) Continue to stir at room temperature for 2 hours, let stand to separate layers, take the water layer, and set aside;
[0037] (3) The aqueous layer solution was extracted 3 times with dichloromethane, and the organic layers were combined, and the organic layers were successively washed with 5% HCl and 5% NaHCO 3 aqueous solution and washed with water to neutral, anhydrous MgSO 4 After drying, filtering, and evaporating the solvent under reduced pressure, 12.6 g of the intermediate compound was obtained with a yield of 83%.
[0038] (4) Dissolve 12.6g of the intermediate compound in 50 mL of acetonitrile, then add 3.7g of N-methylimidazole, heat to reflux for 24 h, distill off the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com