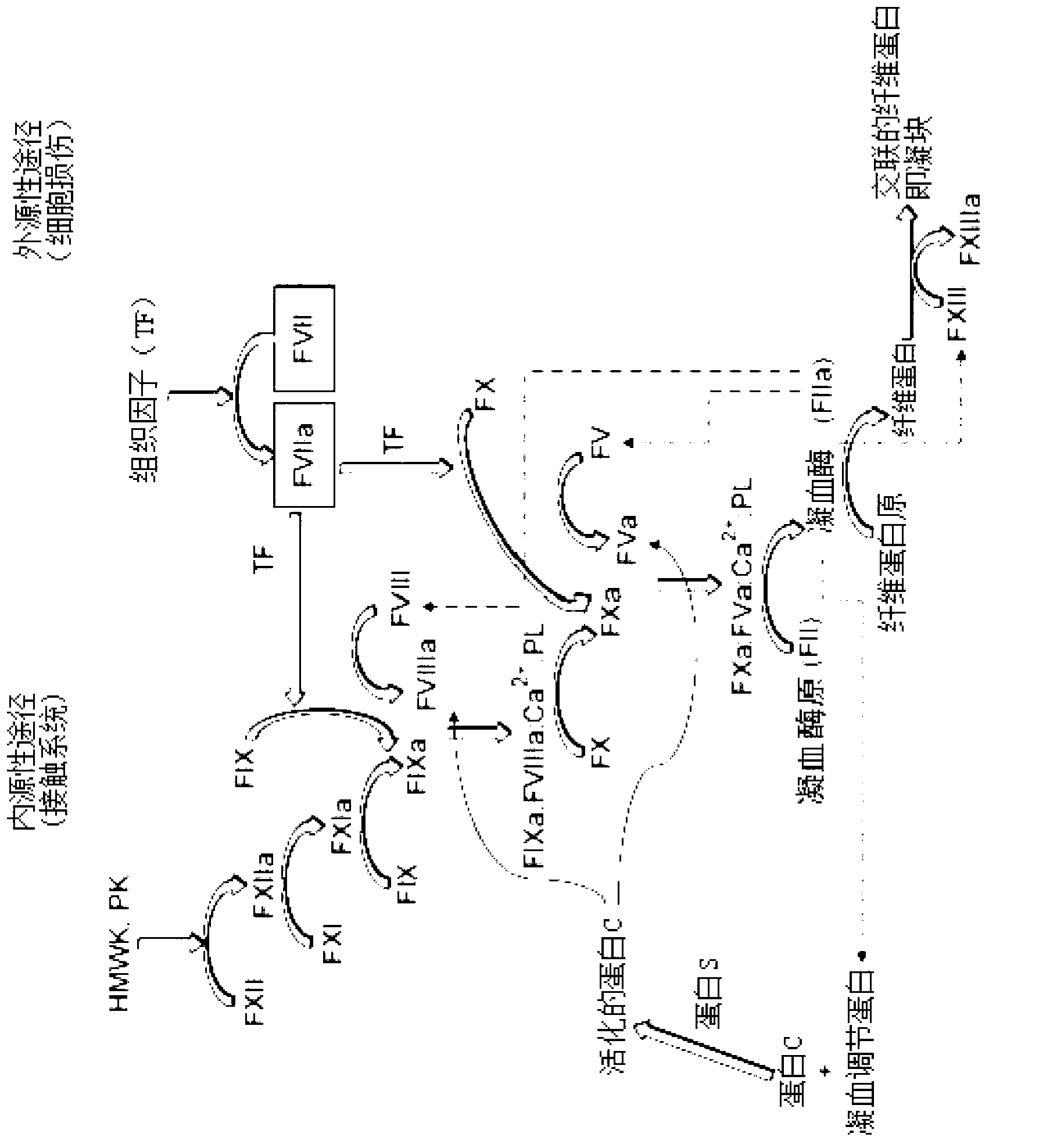
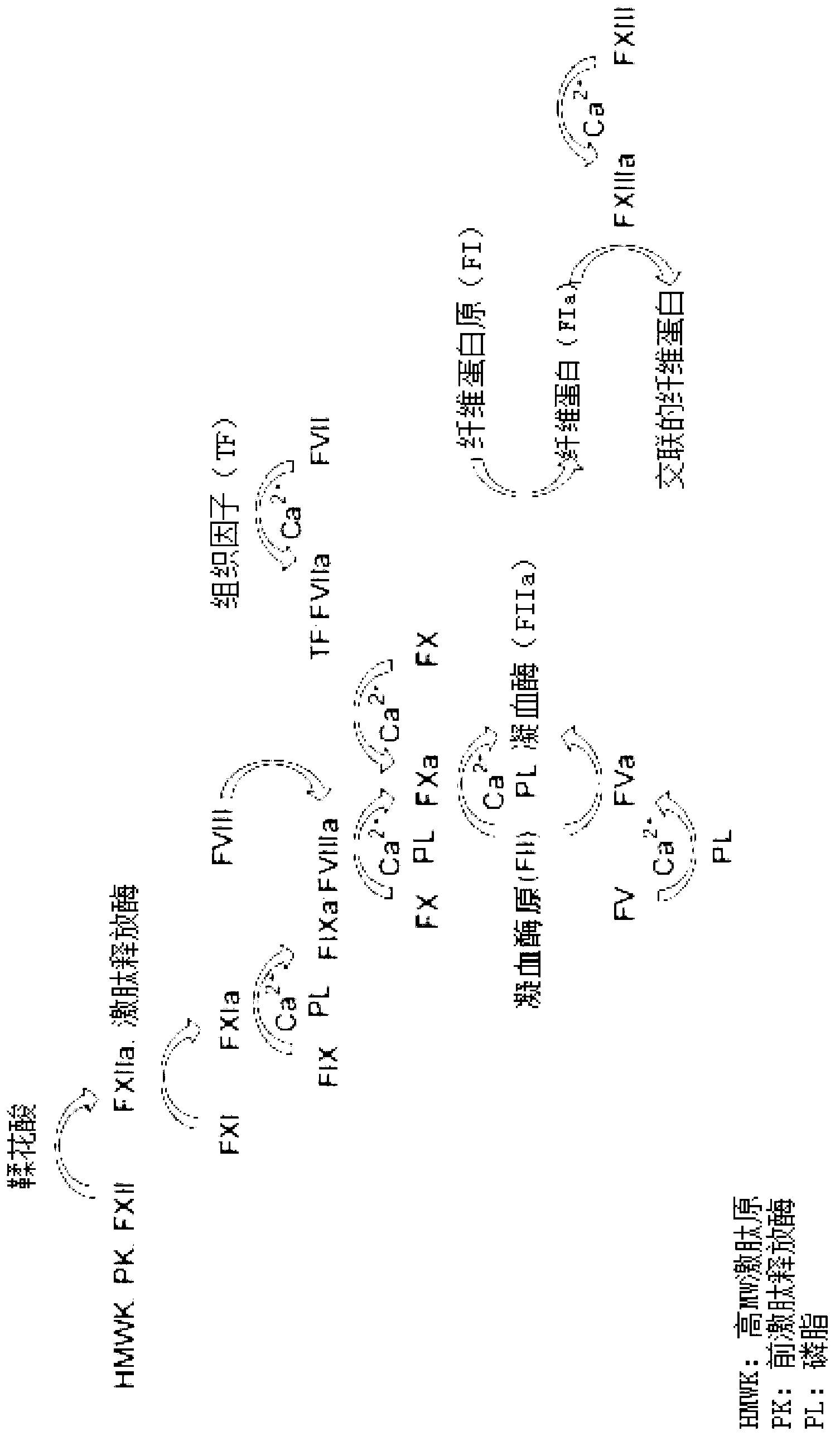
Conjugated blood coagulation factor viii
A kind of FVIII, the technology of conjugation, is applied in the field of coagulation factor VIII of conjugated form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
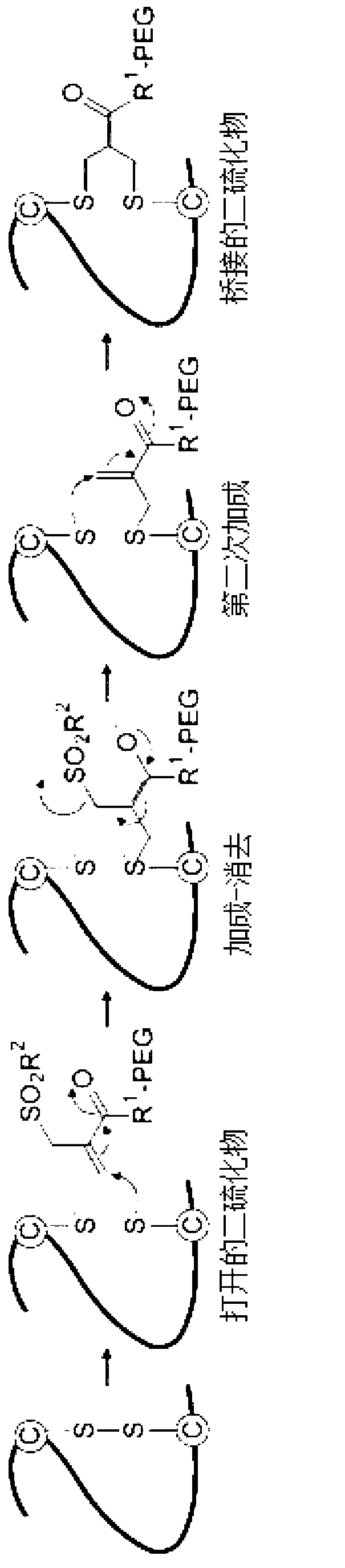
[0131] Example 1: Disulfide PEGylation of FVIII
[0132] Disulfide pegylation of human FVIII was performed according to a modified version of the procedure described by Shaunak et al. in Nat Chem Biol. 2006;2(6):312-313.
Embodiment 2
[0133] Example 2: Reduction of disulfide bonds
[0134] TheraPEG TM The PEGylation process requires the reduction of disulfide bonds. Using a suitable reducing agent such as dithiothreitol (DTT), 2-mercaptoethanol or tris(2-carboxyethyl)phosphine (TCEP), in the presence or absence of selenocystamine (SeCys) For reduction, the concentration of reducing agent used is, for example, 0.5-5 mm DTT or TCEP in low molar excess.
Embodiment 3
[0135] Example 3: PEGylation of FVIII
[0136] TheraPEG for PEGylation of FVIII in small scale reactions (e.g. 5-20 μg of FVIII) TM use for a preliminary assessment. This can help identify conditions under which PEGylated FVIII can be reproducibly prepared using the PEG reactant. Benzamidine or other excipients may be added to prevent proteolysis or to promote protein stabilization.
[0137] Scale up the reaction (eg, 0.2-0.32 mg of FVIII) to produce PEGylated FVIII for initial in vitro testing. A series of PEG-FVIII samples with different PEG molecular weights (eg, 10, 20, and 30 kDa) and different numbers of conjugated PEG moieties (eg, 1-8) were prepared for in vitro analysis.
[0138] The effect of temperature on the PEGylation reaction can be assessed to determine whether temperature affects the conversion of FVIII to PEG-FVIII. However, if preliminary in vitro assessments indicate that higher temperatures (eg, 10-30°C) negatively affect the activity of the pegylate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com