Synthetic method of 2,2-bis (trifluoroethyl) propanol
A bistrifluoroethylpropanol and synthesis method technology, applied in the direction of elimination of hydroxyl groups, organic chemistry, etc., can solve the problems of lack of industrialization prospects in the synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
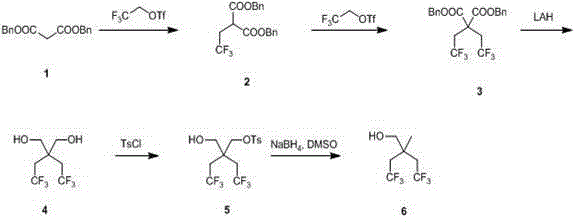
[0021] dibenzyl malonate 1 (40 g, 0.14 mol) was dissolved in 200 mL of anhydrous tetrahydrofuran, cooled to 0 ℃, and sodium hydride (8.5 g, 0.21 mol) was slowly added under nitrogen protection. Stir at 25°C for 30 minutes, then cool to 0°C, add trifluoroethyl trifluoromethanesulfonate (39.2 g, 0.17 mol), stir at 0°C for 10 minutes, stir at 15-25°C, adjust to 1N hydrochloric acid after 12 hours When the pH value reached 5, it was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated, and purified by column with petroleum ether / ethyl acetate at a volume ratio of 30:1 to obtain 2-trifluoroethane as a colorless oil dibenzyl malonate 2 (20 g, yield 39 %).
Embodiment 2
[0023] dibenzyl malonate 1 (40 g, 0.14 mol) was dissolved in 200 mL of anhydrous tetrahydrofuran, cooled to 0 ℃, and sodium hydride (8.5 g, 0.21 mol) was slowly added under nitrogen protection. Stir at 25°C for 30 minutes, then cool to 0°C, add trifluoroethyl trifluoromethanesulfonate (39.2 g, 0.17 mol), stir at 0°C for 10 minutes, reflux at 40-50°C, and cool to 0°C after 12 hours , adjusted the pH value to 5 with 1N hydrochloric acid, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated, and purified by column purification with petroleum ether / ethyl acetate at a volume ratio of 30:1 to obtain a colorless oily Dibenzyl 2-trifluoroethylmalonate 2 (25 g, yield 48%).
Embodiment 3
[0025] dibenzyl malonate 1 (40 g, 0.14 mol) was dissolved in 200 mL of anhydrous tetrahydrofuran, cooled to 0 ℃, and sodium hydride (8.5 g, 0.21 mol) was slowly added under nitrogen protection. Stir at 25°C for 30 minutes, then cool to 0°C, add trifluoroethyl trifluoromethanesulfonate (39.2 g, 0.17 mol), stir at 0°C for 10 minutes, reflux at 40-50°C, and cool to 0°C after 36 hours , adjusted the pH value to 5 with 1N hydrochloric acid, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated, and purified by column purification with petroleum ether / ethyl acetate at a volume ratio of 30:1 to obtain a colorless oily Dibenzyl 2-trifluoroethylmalonate 2 (40 g, yield 78%).
[0026] H NMR spectrum CDCl 3 400MHz,d 7.28-7.38 (10H, m, Ar-H), 5.12-5.22 (4H, Bn-H), 3.77-3.80 (1H, t, -CH-), 2.83-2.92 (2H, m, -CH 2 -CF 3 ).
[0027] 2,2-Dibenzyl trifluoroethyl malonate 3 synthesis
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com