Preparation method for 6-carboxylfluorescein
A technology of carboxyfluorescein and carboxyl group, applied in the field of preparation of 6-carboxyfluorescein, can solve problems such as large loss and low yield, and achieve the effects of reducing production cost, high application value, and simplifying purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] a. Reaction steps:
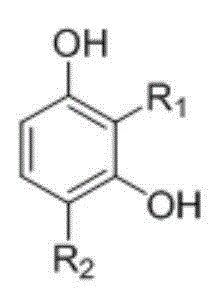
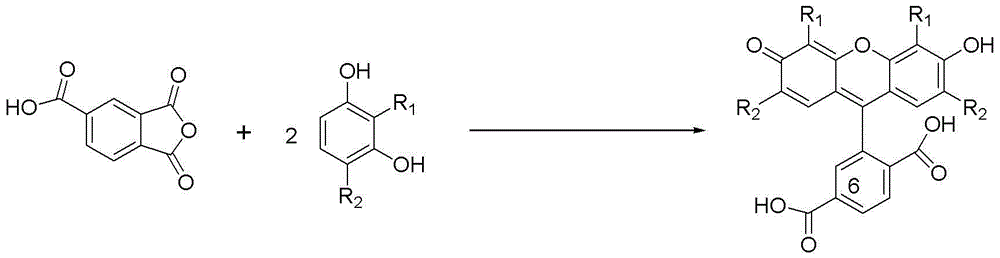
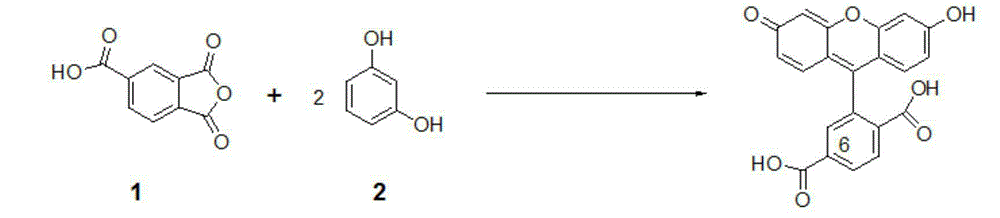
[0022] At room temperature, in a reaction vessel, the resorcinol 2 (resorcinol, 57.2 g, 0.52 mol) dissolved in methanesulfonic acid (520 ml), added trimellitic anhydride 1 (1,2,4-Benzenetricarboxylic anhydride, 50 g, 0.26 mol), after stirring at room temperature for 10 minutes, tin tetrachloride (SnCl 4 , 6.1 ml, 0.055 mol), heated, and reacted at 90 ° C. After 6 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction system was cooled to room temperature to obtain a reaction solution;
[0023] b. Crystallization step
[0024] The reaction solution was poured into 4 liters of ice-water solution, stirred for 10 minutes, and the product precipitated out. Filter and dry, and the dried crude product is crystallized with methanol and hexane (methanol:hexane volume ratio is 1:4). Filter and dry to obtain 6-carboxyfluorescein methylsulfonic acid adduct.
[0025] c, hydrolysis step:
[0026] Add 6-ca...
Embodiment 2
[0031]
[0032] a. Reaction steps:
[0033] At room temperature, in a reaction vessel, 4-chlororesorcinol 3 (4-Chlororesorcinol, 75.2 g, 0.52 mol) dissolved in methanesulfonic acid (620 ml), adding trimellitic anhydride 1 (1,2,4-Benzenetricarboxylic anhydride, 50 g, 0.26 mol), after stirring at room temperature for 15 minutes, tin tetrachloride (SnCl 4 , 6.7 ml, 0.057 mol), heated, and reacted at 105 °C. After 6 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction system was cooled to room temperature to obtain a reaction solution;
[0034] b. Crystallization step
[0035] The reaction solution was poured into 4 liters of ice-water solution, stirred for 10 minutes, and the product precipitated out. Filter and dry, and the dried crude product is crystallized with methanol and hexane (methanol:hexane volume ratio is 1:4). Filter and dry to obtain 2',7'-dichloro-6-carboxyfluorescein methanesulfonic acid adduct.
[0036] c, hydrol...
Embodiment 3
[0042]
[0043] a. Reaction steps:
[0044] At room temperature, in a reaction vessel, 4-methylresorcinol 4 (4-methylresorcinol, 64.6 g, 0.52 mol) was dissolved in methanesulfonic acid (600 ml), and trimellitic anhydride was added 1 (1,2,4-Benzenetricarboxylic anhydride, 50 g, 0.26 mol), after stirring at room temperature for 10 minutes, tin tetrachloride (SnCl 4, 5.8 ml, 0.052 mol), heated, and reacted at 95 ° C. After 6 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction system was cooled to room temperature to obtain a reaction solution;
[0045] b. Crystallization step
[0046] The reaction solution was poured into 4 liters of ice-water solution, stirred for 10 minutes, and the product precipitated out. Filter and dry, and the dried crude product is crystallized with methanol and hexane (methanol:hexane volume ratio is 1:4). Filter and dry to obtain 2',7'-dimethyl-6-carboxyfluorescein methanesulfonic acid adduct.
[0047]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com