Preparation method of nitrile compound
A compound and nitrile technology, applied in the field of preparation of nitrile compounds and one-pot synthesis of heterocyclic compounds, can solve the problems of complicated operation, poor selectivity, long synthesis steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
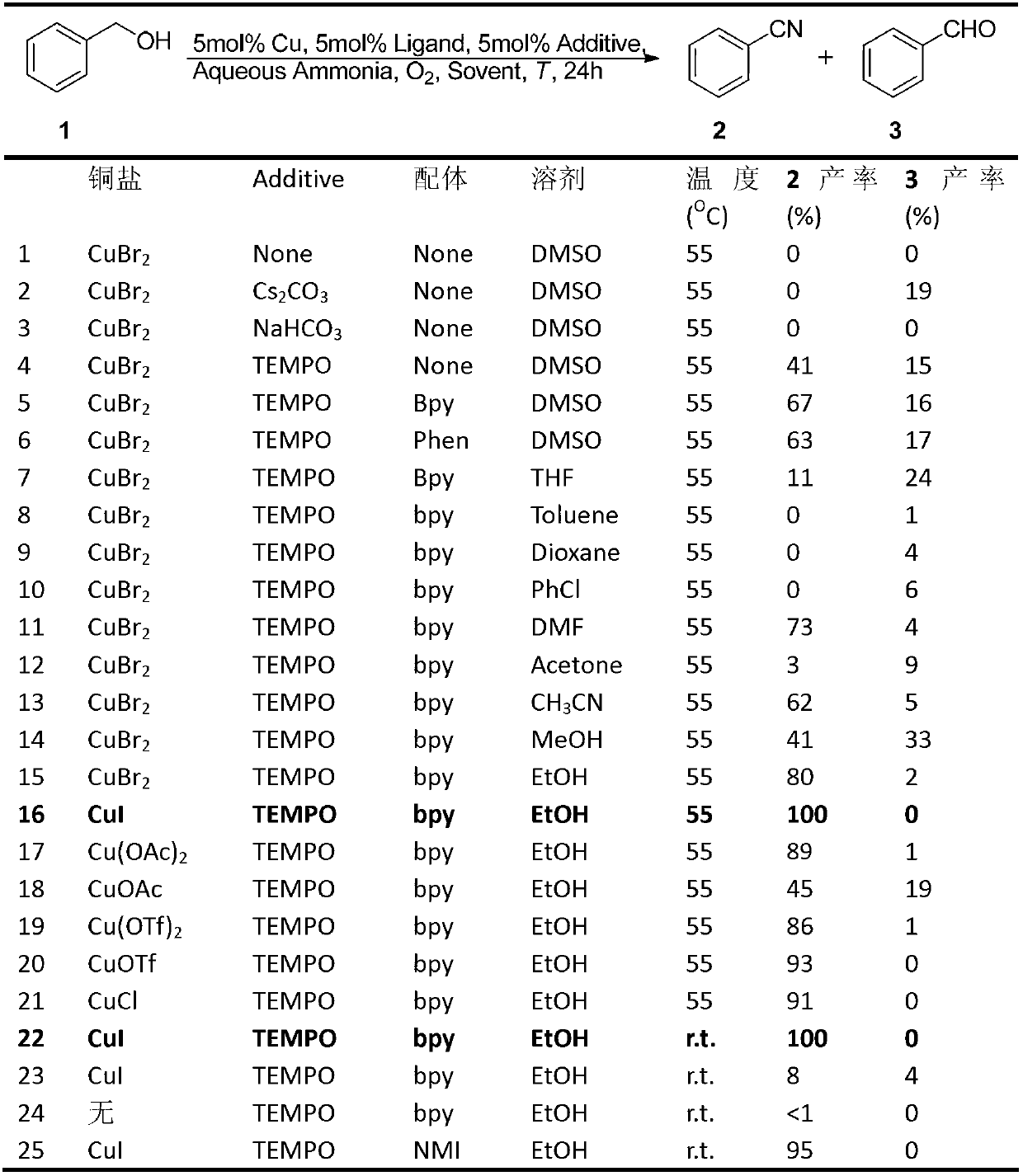
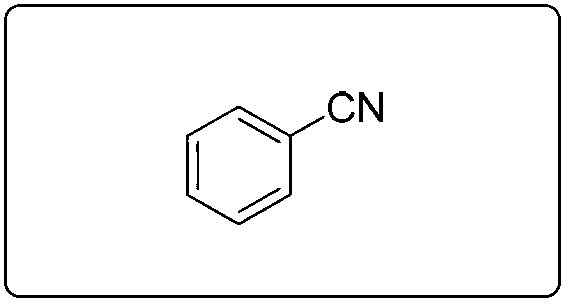
[0084] Embodiment 1. Preparation and characterization of benzonitrile
[0085]
[0086] Add 9.5mg (0.05mmol) of cuprous iodide, 7.8mg (0.05mmol) of bipyridine, and 7.8mg (0.05mmol) of tetramethylpiperidine oxide into a dry 10mL schlenk bottle, 2mL of anhydrous EtOH, oxygen replacement Three times, slowly add 1.0mmol benzyl alcohol, 150uL ammonia water (2.0mmol, 2.0eq), stir at room temperature for 24h, monitor the reaction by TLC, after the reaction is completed, separate by column chromatography to obtain the target product, a colorless liquid, with a yield of 97%. 1 H NMR (400MHz, CDCl 3 )δ7.68 7.55(m,3H),7.47(t,J=7.7Hz,2H); 13 C NMR (100MHz, CDCl 3 )δ132.82,132.16,129.16,118.86,112.43; MS(70eV):m / z(%)103.1(M+,100).
Embodiment 2
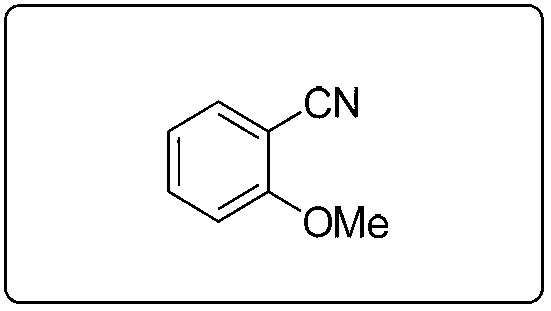
[0087] Preparation and characterization of embodiment 2.2-methoxybenzonitrile
[0088]
[0089] Using 2-methoxybenzyl alcohol (1.0 mmol), the title compound was prepared by a method similar to that described in Example 1 as a yellow oily liquid with a yield of 99%. 1 H NMR (400MHz, CDCl 3 )δ7.567.51(m,2H),7.01 6.96(m,2H),3.92(s,3H); 13 C NMR (100MHz, CDCl 3 )δ161.17, 134.41, 133.66, 120.72, 116.49, 111.28, 101.64, 56.96; MS (70eV): m / z (%) 133.1 (M + ,100).
Embodiment 3
[0090] Preparation and characterization of embodiment 3.3-methoxybenzonitrile
[0091]
[0092] Using 3-methoxybenzyl alcohol (1.0 mmol), the title compound was prepared by a method similar to that described in Example 1 as a colorless oily liquid with a yield of 90%. 1 H NMR (400MHz, CDCl 3 )δ7.417.37(m,1H),7.27 7.25(m,1H),7.16 7.14(m,2H),3.85(s,3H); 13 C NMR (100MHz, CDCl 3 )δ159.62, 130.35, 124.50, 119.33, 118.73, 116.85, 113.18, 55.59; MS (70eV): m / z (%) 133.1 (M + ,100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com