Diclofenac salt of tramadol
A technology of diclofenac and tramadol, applied in the preparation of organic compounds, active ingredients of anhydride/acid/halide, medical preparations containing active ingredients, etc., can solve the problem of difficult to obtain diclofenac aqueous injection solution, low solubility, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1. Obtaining the method for tramadol-diclofenac (1: 1) co-crystal
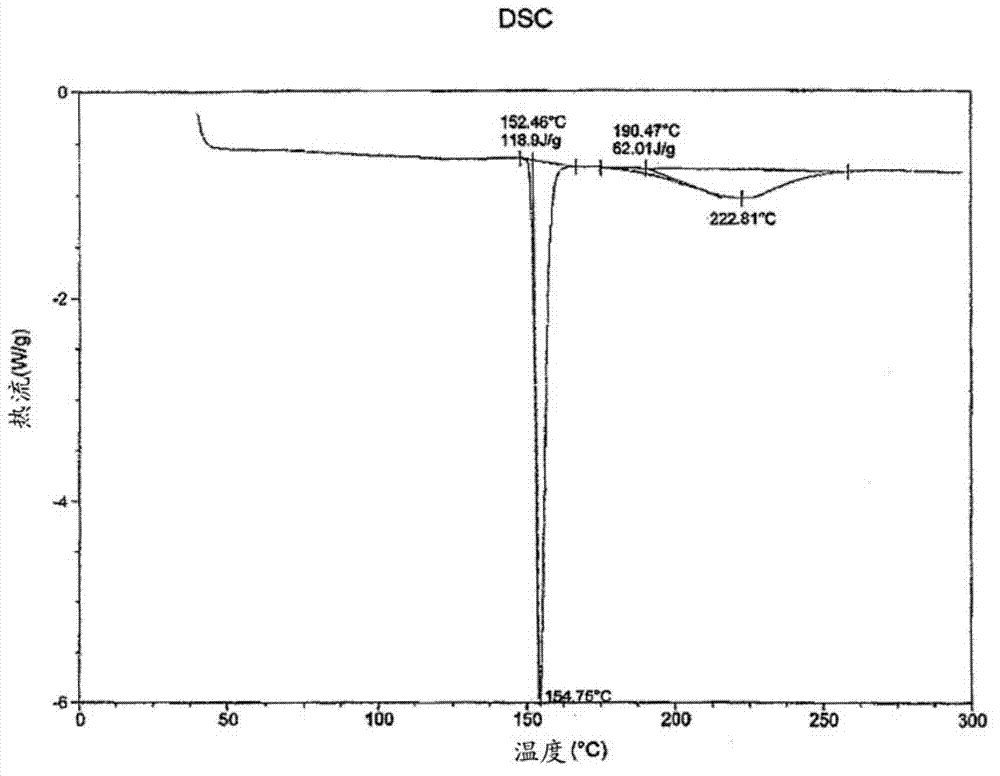
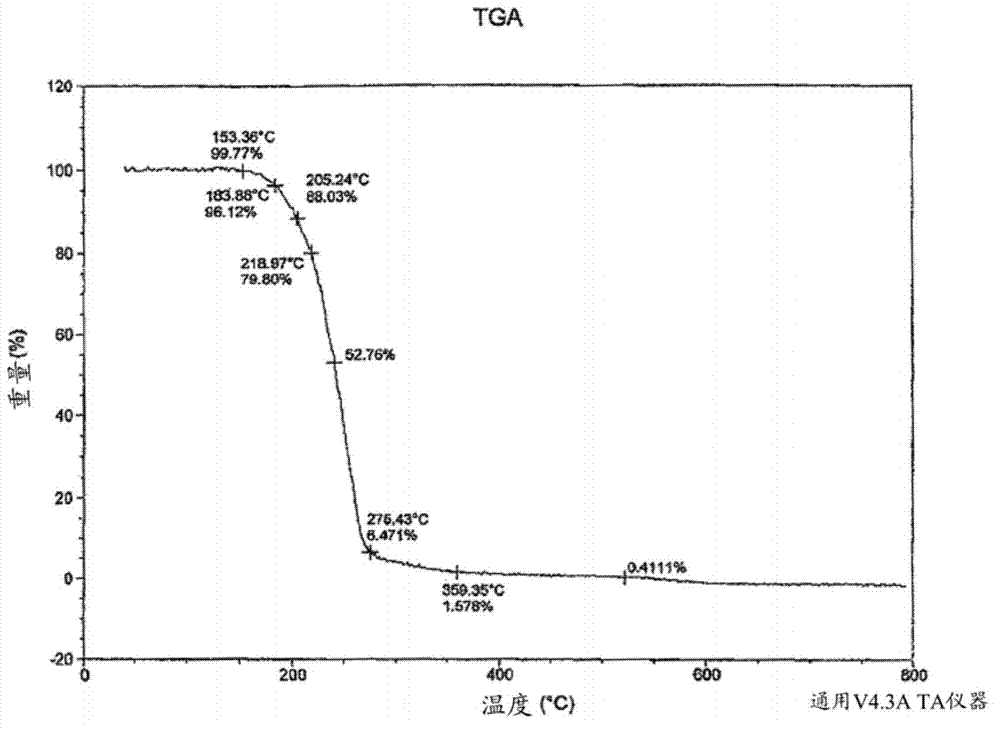
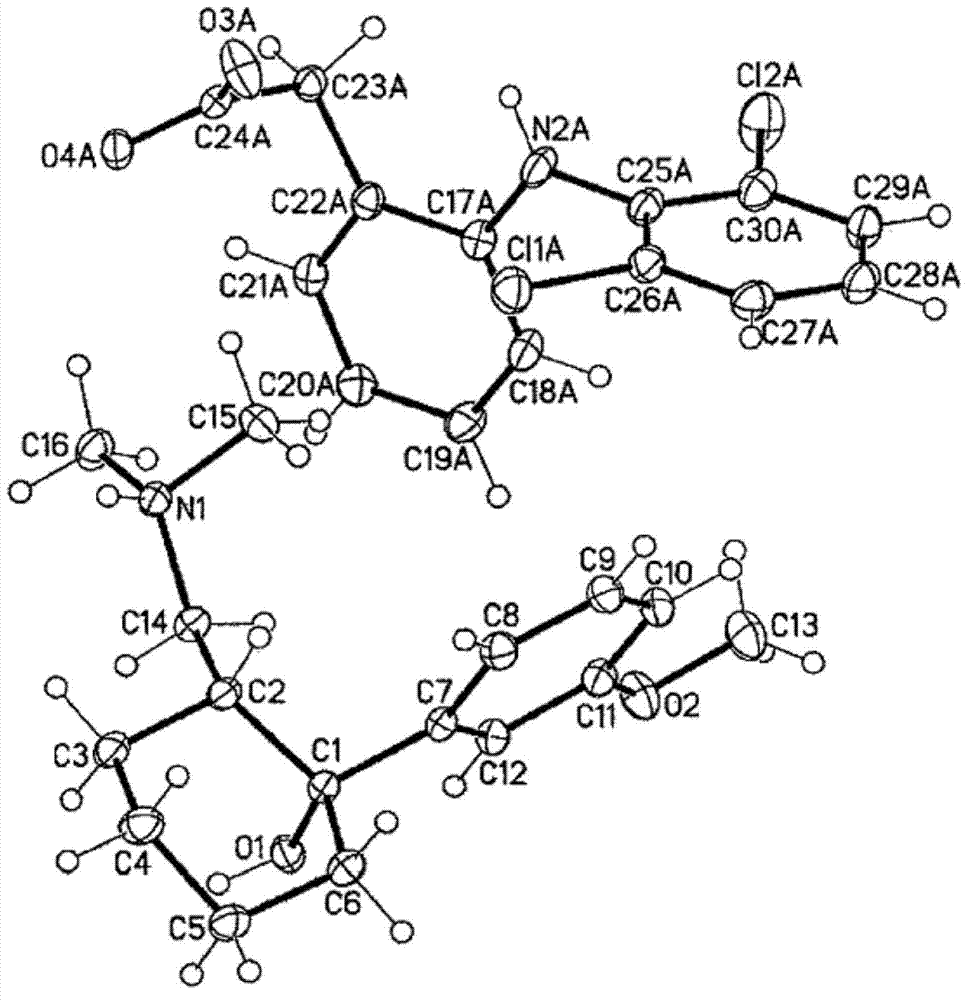
[0080] A solution of tramadol free base (22 g, 83.5 mmol) in 50 mL of dichloromethane was added to a stirred solution of diclofenac free acid (24.7 g, 83.4 mmol) in 50 mL of ethyl acetate. The mixture was heated until dissolved, then the solvent was evaporated under vacuum to yield a white amorphous solid. The amorphous solid was dissolved in 350 mL of acetonitrile, then heated to 75°C with stirring for 1 hour. The supernatant was cooled to room temperature to produce a white precipitate. The obtained precipitate was filtered off and dried by oven to afford a crystalline white solid (43.6 g, 93.2% yield) of diclofenac salt with a 1:1 ratio of tramadol.
[0081] The crystalline form of the diclofenac salt of tramadol was characterized by a powder XRD profile having X-ray powder diffraction peaks selected from the group consisting of: at about 11.0°, 19.0°, 20.5° and 20.8° ± 0.2° 2Θ. In this...
Embodiment 2
[0082] Example 2. Identification of Diclofenac Salt (1:1) of Tramadol
[0083] The diclofenac salt of tramadol of Example 1 (1:1 ratio) was identified by the following analysis.
[0084] NMR
[0085] The proton nuclear magnetic resonance (NMR) analysis record of the sample of embodiment 1 is by Bruker Avance II 400Ultrashield NMR in deuterated chloroform (CDCl 3 ) is obtained.
[0086] Diclofenac with Tramadol: 1 H NMR (400MHz, CDCl 3 )δ8.12 (br, 1H, proton of protonated tertiary amine), 7.31 (d, J=8.0Hz, 1H), 7.25 (t, J=8.0Hz, 1H), 7.22 (dd, J=6.0, 1.6 Hz, 1H), 7.09(br, 1H), 7.03(td, J=7.6, 1.6Hz, 1H), 6.98(d, J=8.0Hz, 1H), 6.93(t, J=8.0Hz, 1H), 6.86(td, J=7.6, 1.2Hz, 1H), 6.75(dd, J=8.0, 2.4Hz, 1H), 6.49(dd, J=7.6, 1.2Hz, 1H), 3.80(s, 3H), 3.75( s, 2H), 2.72(dd, J=13.2, 8.0Hz, 1H), 2.29(s, 6H), 2.25(dd, J=13.2, 2.0Hz, 1H), 1.94-2.00(m, 1H), 1.82 -1.86(m, 1H), 1.52-1.79(m, 6H), 1.24-1.31(m, 1H) 13 C NMR (100MHz, CDCl 3 )δ177.64,159.63,149.65,142.88,138.33,130.70,129....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com