Arsenic trioxide controllable-releasing balloon and preparing method thereof
An arsenic trioxide and balloon technology, which is used in balloon catheters, drug devices, and other medical devices, etc., can solve problems such as drug loss, uneven administration, and impact on product treatment effects, and achieve increased adsorption capacity and reduced loss rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be described in further detail below in conjunction with the embodiments and accompanying drawings, but the embodiments of the present invention are not limited thereto.
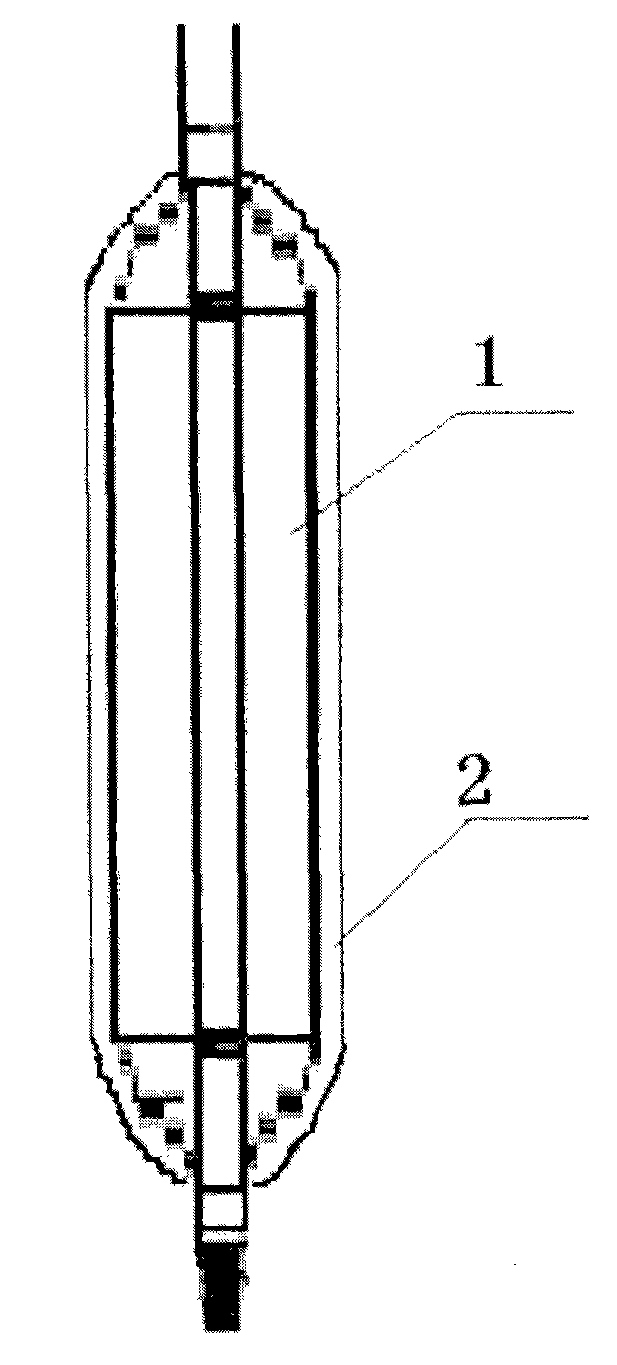
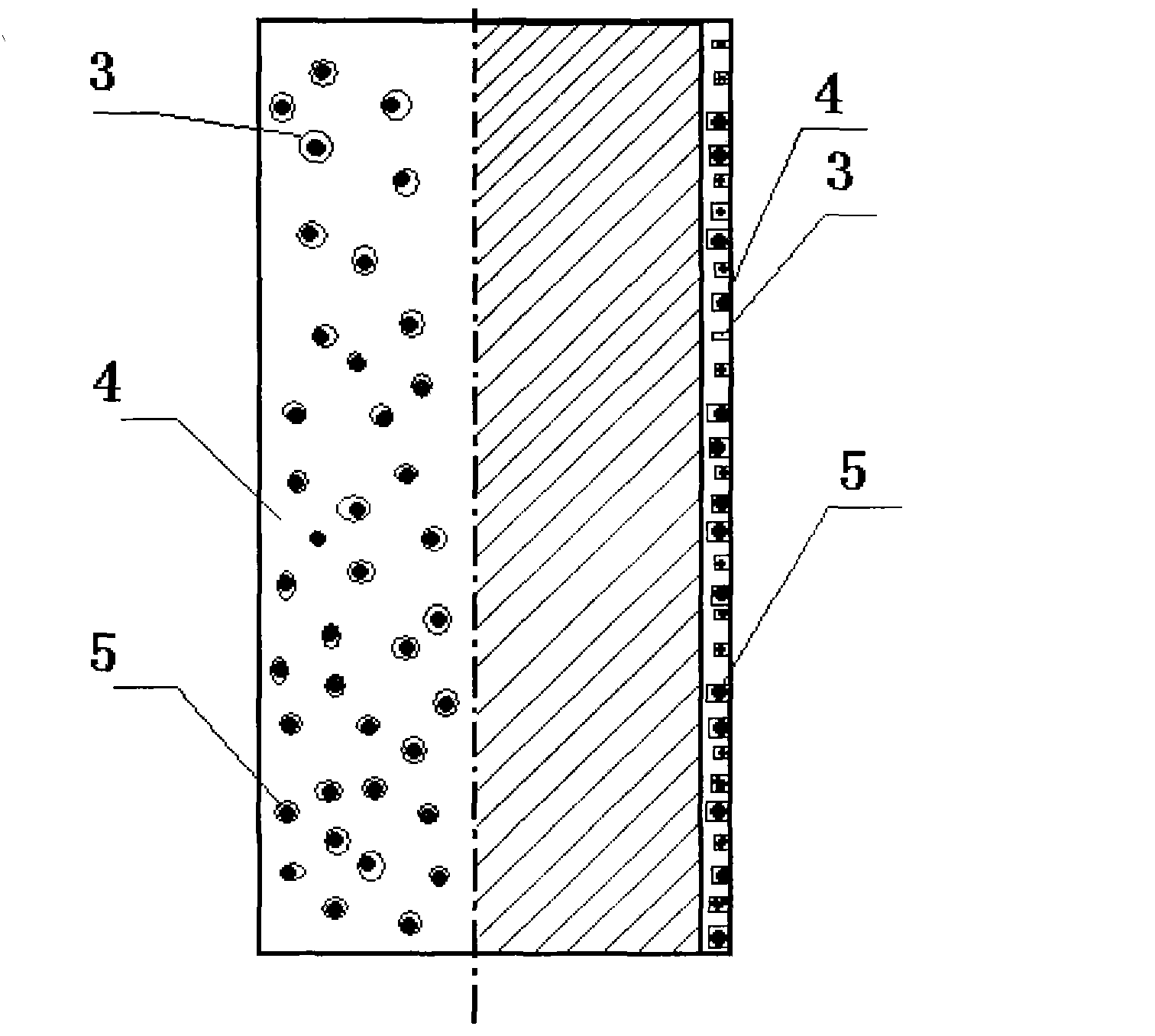
[0025] see Figure 2 to Figure 3As shown, the outer surface of the arsenic trioxide controllable release balloon of the present invention is coated with a polymer coating, and the surface of the polymer coating has a concave hole structure, which is obtained by piercing with a special concave hole needle. The holes are filled with the arsenic trioxide drug particles, so that the arsenic trioxide drug particles or independent particle clusters are like "islands", so that the drug can be released in a controlled manner.
[0026] A method for preparing the above-mentioned arsenic trioxide controllable release balloon, which uses a spraying method to spray a polymer coating on the outer surface of the balloon. The diameter of the hole is 1-50um, and the depth of the concave hol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com