IAP BIR domain binding compounds
A compound, alkyl technology, applied in the field of IAP BIR domain binding compounds, can solve the problem of no RING domain and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
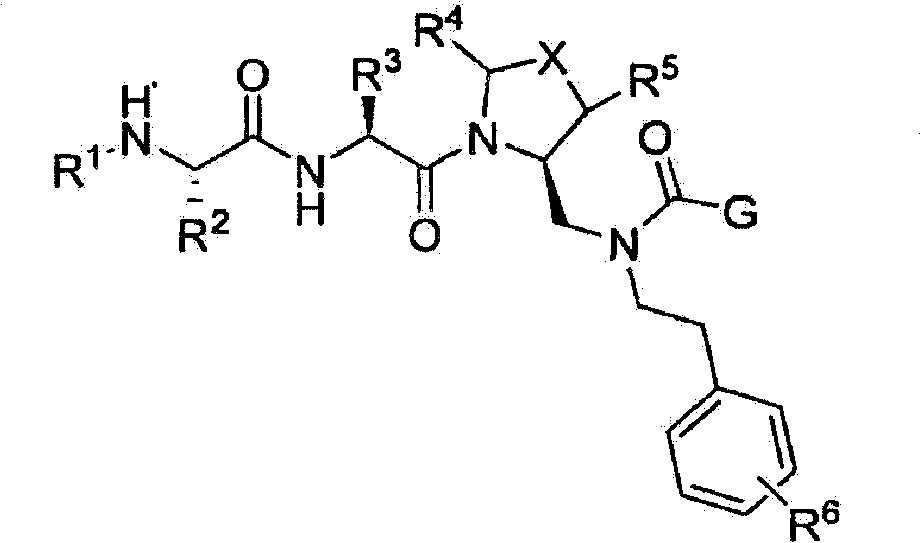
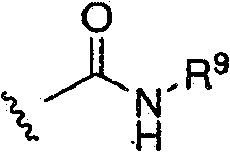
[0036] According to one embodiment, G is:
[0037]
[0038] Or substituted or unsubstituted pyrrole, specific examples of which include but are not limited to:
[0039]
[0040] Or substituted or unsubstituted imidazole, specific examples thereof include but are not limited to:
[0041]
[0042] Or substituted or unsubstituted pyrazole, specific examples thereof include but are not limited to:
[0043]
[0044] Or substituted or unsubstituted triazoles, specific examples of which include but are not limited to:
[0045]
[0046] Or substituted or unsubstituted thiazole, specific examples of which include but are not limited to:
[0047] Where R 11 NHC(O)CH 3 Or phenyl;
[0048] Or substituted or unsubstituted tetrazole, specific examples of which include but are not limited to:
[0049]
[0050] Or substituted or unsubstituted oxazoles, specific examples of which include but are not limited to:
[0051]
[0052] Or substituted or unsubstituted isoxazole, specific examples thereof include b...
Embodiment 1
[0247] The following examples illustrate the preparation of compound 5-e, which can be used as an intermediate in the preparation of the compound of formula 1 or its salt.
[0248] Route 5: Synthesis of Intermediate 5-e
[0249]
[0250] Step 1: Add DIPEA (10.33mL, 59.3mmol), HOBt (4.81g, 35.6mmol) and HBTU (13.50) to the DMF solution of Boc-Chg-OH (9.16g, 35.6mmol) cooled to 0°C.
[0251] g, 35.6 mmol). After stirring for 10 minutes, (S)-prolinol (3.0 g, 29.7 mmol) was added and the reaction mixture was stirred overnight at room temperature. Add water and ethyl acetate, separate the organic layer, use 10% citric acid, NaHCO 3 Aqueous and brine washing, anhydrous MgSO 4 Dry, filter and concentrate in vacuo. Purification by silica gel chromatography provided intermediate 5-a as a colorless oil.
[0252] Step 2: To Intermediate 5-a (10.10 g, 29.7 mmol) was added 1,4-dioxane (30 mL) containing 4N HCl and the solution was stirred at room temperature for 1 hour. The volatiles were remov...
Embodiment 2
[0257] The following example illustrates the preparation of compound 6-h, which can be used as an intermediate in the preparation of the compound of formula 1 or its salt.
[0258] Route 6: Synthesis of Intermediate 6-h
[0259]
[0260] Step 1: To a dichloromethane solution (300 mL) of N-(tert-butoxycarbonyl)-L-prolinal 6-a (10.0 g, 50.2 mmol) was added phenethylamine (6.52 mL, 50.2 mmol). After stirring for 2 hours at room temperature, the reaction was cooled to 0°C, sodium triacetoxyborohydride (21.0 g, 100.3 mmol) was added portionwise and then the reaction mixture was stirred at room temperature overnight. Add 10% Na 2 CO 3 Aqueous solution, separate the organic layer, extract the aqueous phase with dichloromethane, wash the combined organic extracts with brine, anhydrous MgSO 4 Dry, filter and concentrate in vacuo to provide intermediate 6-b as a colorless oil. Intermediate 6-b was dissolved in diethyl ether (125 mL), the solution was cooled to 0° C. and 1N HCl in diethyl et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com