Bromfenac sodium hydrate eye drops and preparation method thereof
A technology of bromfenac sodium and hydrate, which is applied in the field of eye drops and its preparation, can solve problems such as eye irritation, instability in water, and instability of bromfenac sodium, and achieve low incidence of side effects and good healing effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
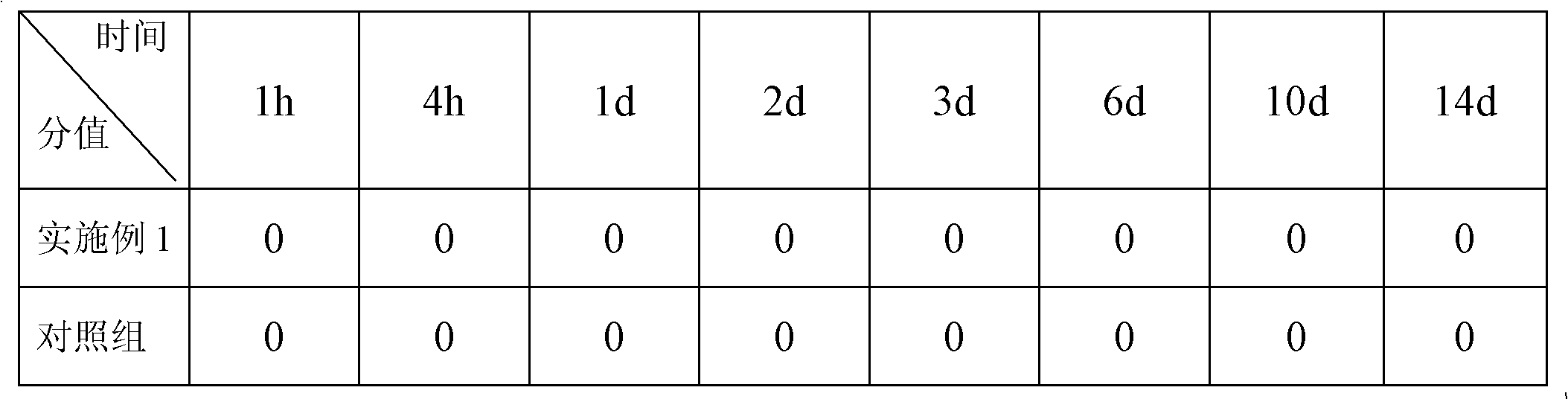
Embodiment 1
[0016] Add 3000 g of water for injection into the concentrated preparation tank, and at the same time add 10.35 g of bromfenac sodium hydrate, 15 g of Tween 80, 2 g of disodium edetate, 20 g of anhydrous sodium sulfite, and 1 g of benzalkonium bromide, stir until uniform, and statically After standing for 30 minutes, it is filtered through a filter, and the filtrate is sent to the dilution tank;
[0017] Add 3000g of water for injection into the dilute tank, add 0.5g of boric acid, 0.5g of borax, and 200g of povidone at the same time, continue to add water to make up to 10000g, add sodium hydroxide to adjust the pH of the system to 8.0, and continue stirring for 20 minutes until the liquid is uniform ;
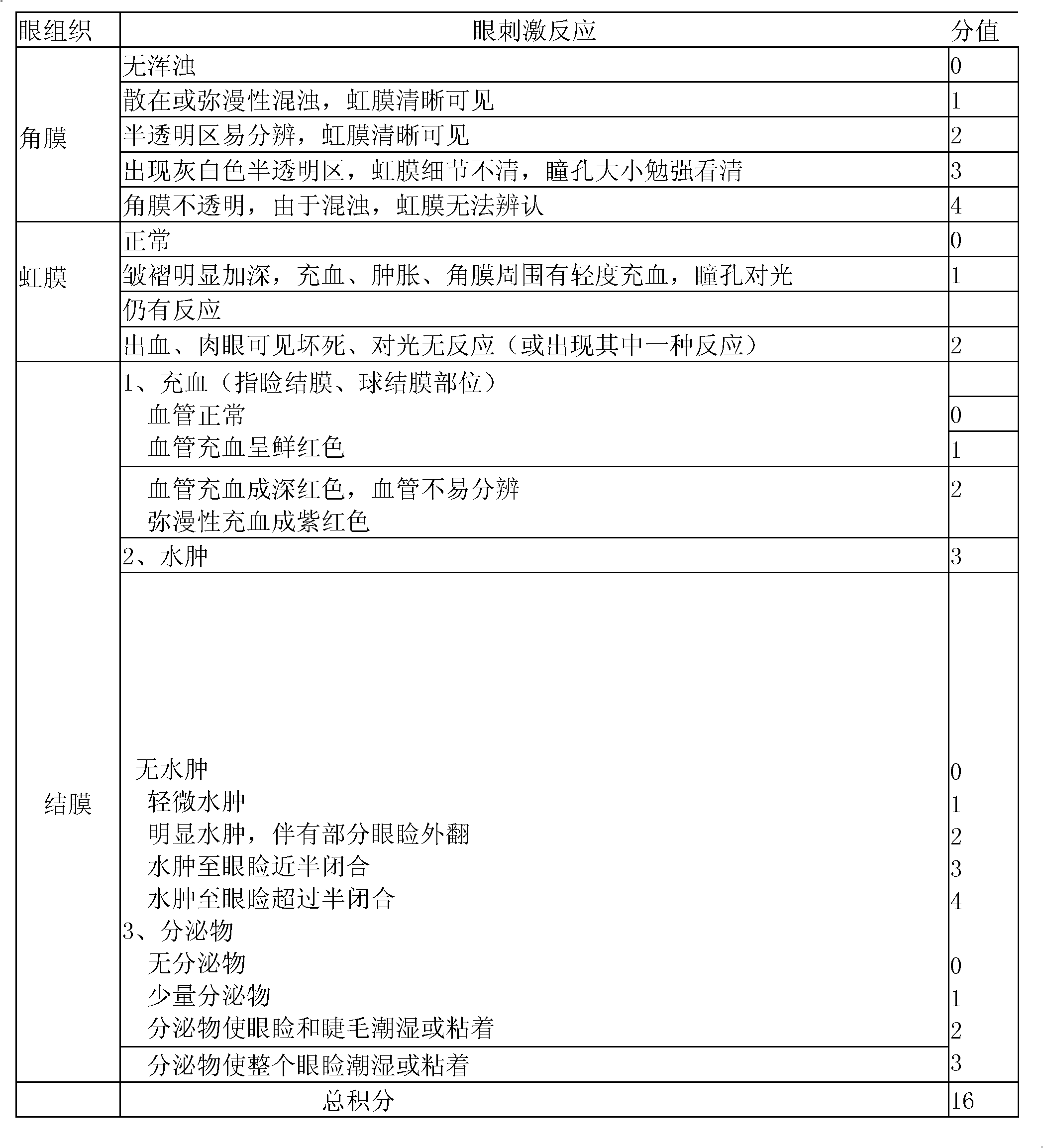
[0018] (3) Inspection, filter sterilization, potting, light inspection, packaging, full inspection, filter sterilization process uses a microporous membrane with a pore size of 0.45 μm.
Embodiment 2
[0020] Add 2000g of water for injection into the concentrated preparation tank, and at the same time add 10g of bromfenac sodium hydrate, 10g of Tween 80, 3g of disodium edetate, 40g of anhydrous sodium sulfite, and 2g of benzalkonium bromide, stir until uniform, and let stand After 40 minutes, filter through the filter, and the filtrate is sent to the dilution tank;
[0021] Add 3000g of water for injection into the dilute preparation tank, add 1g of boric acid, 2g of borax, and 400g of povidone at the same time, continue to add water to make up to 10000g, add sodium hydroxide to adjust the pH of the system to 8.4, and continue stirring for 20 minutes until the liquid medicine is uniform;
[0022] (3) Inspection, filter sterilization, potting, light inspection, packaging, full inspection, filter sterilization process uses a microporous membrane with a pore size of 0.22 μm.
Embodiment 3
[0024] Add 2500g of water for injection into the concentrated preparation tank, add 20g of bromfenac sodium hydrate, 30g of Tween 80, 2g of disodium edetate, 50g of anhydrous sodium sulfite, and 1g of benzalkonium bromide, stir until uniform, and let stand After 60 minutes, filter through the filter, and send the filtrate to the dilution tank;
[0025] Add 2500g of water for injection into the dilute preparation tank, add 2g of boric acid, 1.5g of borax, and 100g of povidone at the same time, continue to add water to make up to 10000g, add sodium hydroxide to adjust the pH of the system to 8.6, and continue stirring for 20 minutes until the liquid medicine is uniform;
[0026] (3) Inspection, filter sterilization, potting, light inspection, packaging, full inspection, filter sterilization process uses a microporous membrane with a pore size of 0.22 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com