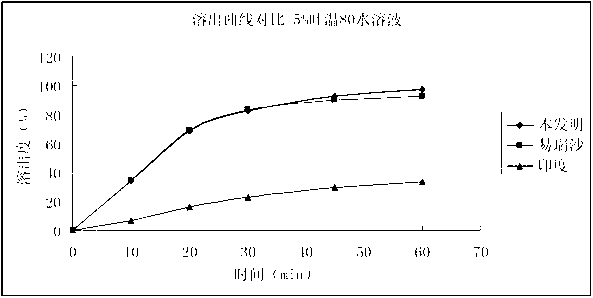
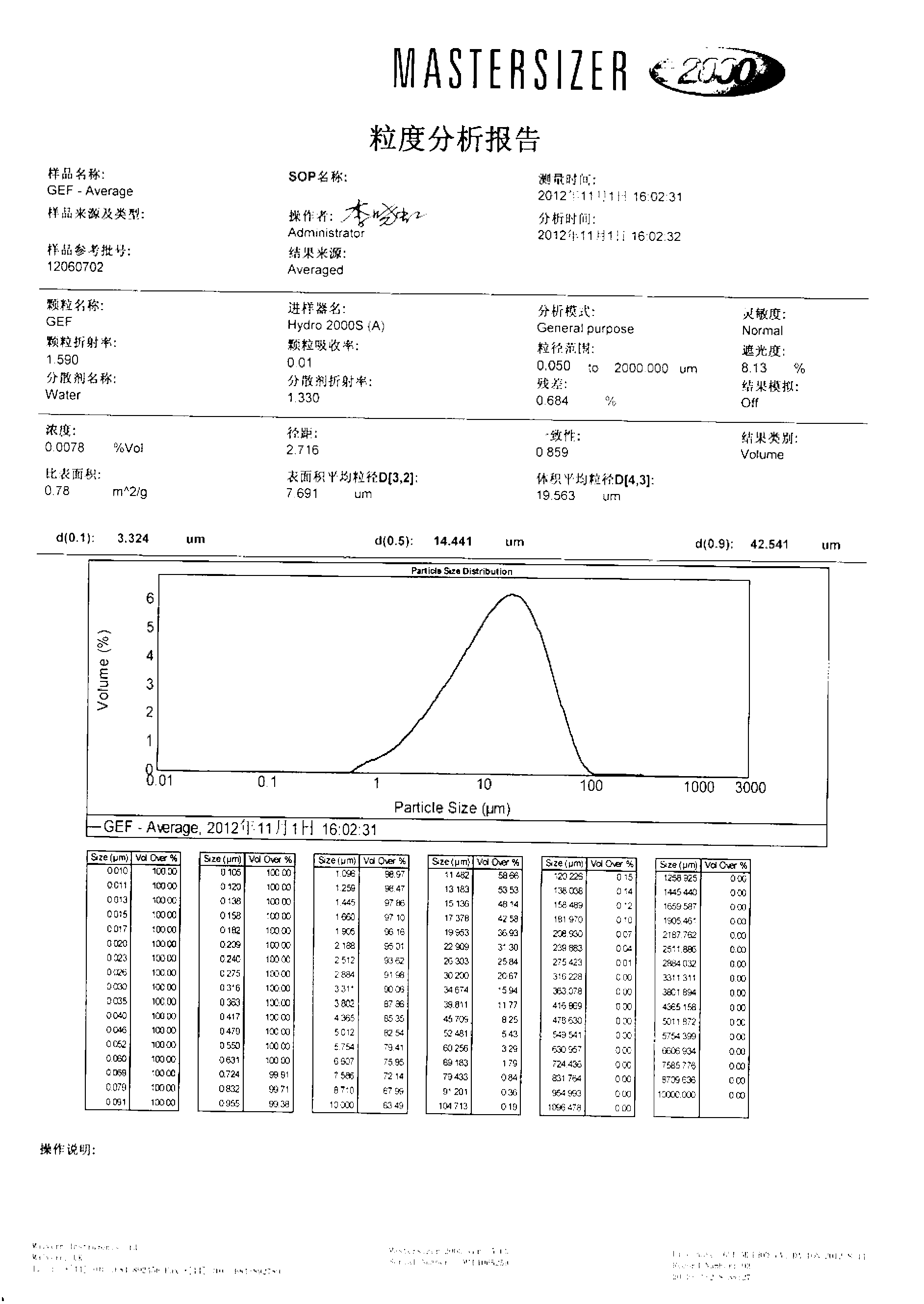
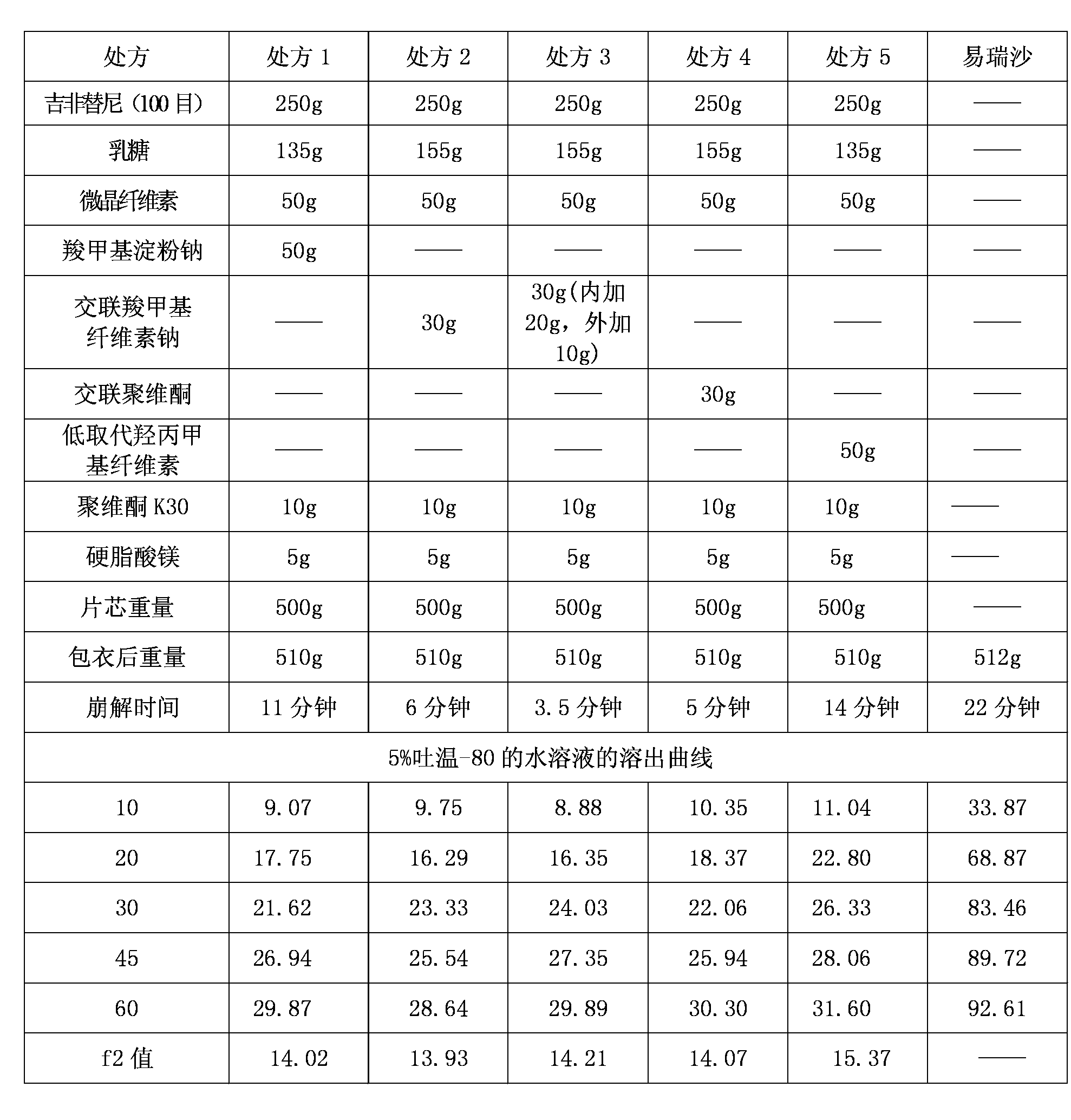
Drug composition containing gefitinib
A gefitinib and composition technology, which is applied in the field of tablets containing gefitinib, can solve the problems such as changes in the dissolution profile, unsatisfactory stability, and inability to achieve therapeutically effective concentrations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Prescription (1000 tablets):
[0080] Gefitinib: 250.0g Lactose: 163.5g
[0081] Microcrystalline Cellulose: 50.0g Croscarmellose Sodium: 20.0g
[0082] Povidone K 30 : 10.0g Sodium lauryl sulfate: 1.5g
[0083] Magnesium stearate: 5.0g Gastric film coating premix: 12.5g
[0084] The particle size distribution of gefitinib is: D(0.1)=3μm, D(0.5)=13μm, D(0.9)=40μm.
Embodiment 2
[0086] The preparation process of embodiment 1: tablet core: earlier auxiliary material (that is, the material other than gefitinib) is passed through 80 mesh sieves respectively, standby; Get the gefitinib of prescription quantity, lactose, microcrystalline cellulose, twelve Sodium alkyl sulfate and croscarmellose sodium, mixed for about 15 minutes to make it uniform; Povidone K 30 Dissolve in water to make a binder solution with a concentration of 8%, add it to the above mixed powder, stir, and prepare soft materials; make granules with 14 mesh sieves; dry the wet granules at 60°C±5°C until the moisture content is lower than 2 %; The dry granules are sieved through a 20-mesh sieve, added with the prescribed amount of magnesium stearate, mixed evenly, and compressed into tablets.
[0087] Coating: adding water to the stomach-soluble film coating premix to make a coating solution with a concentration of 18%, the coating solution passes through a 100-mesh sieve to coat the tabl...
Embodiment 3
[0089] The types and dosages of raw and auxiliary materials for the prescription (1000 tablets) are the same as in Example 1, except that the particle size distribution of gefitinib is: D(0.1)=2μm, D(0.5)=18μm, D(0.9)=35μm.
[0090] Technology: with embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com