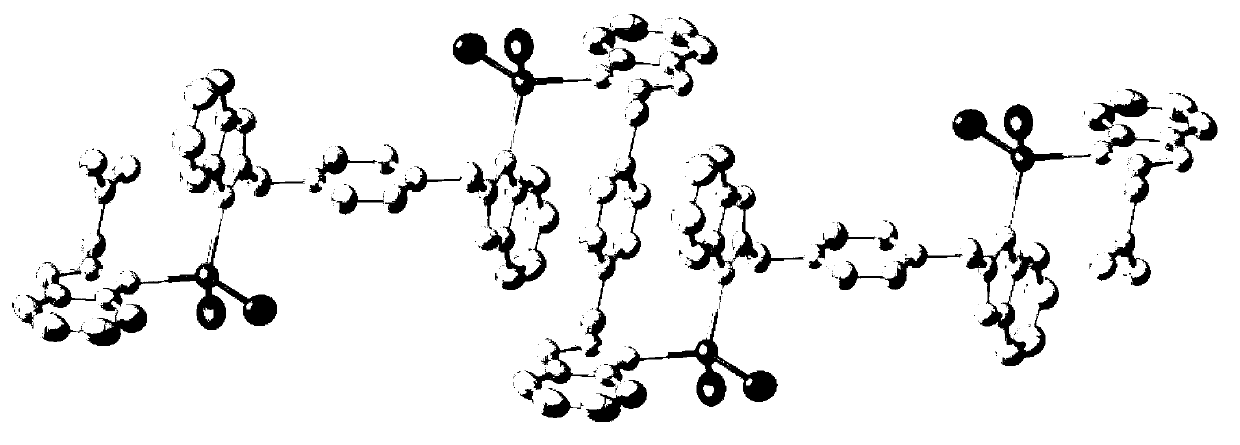
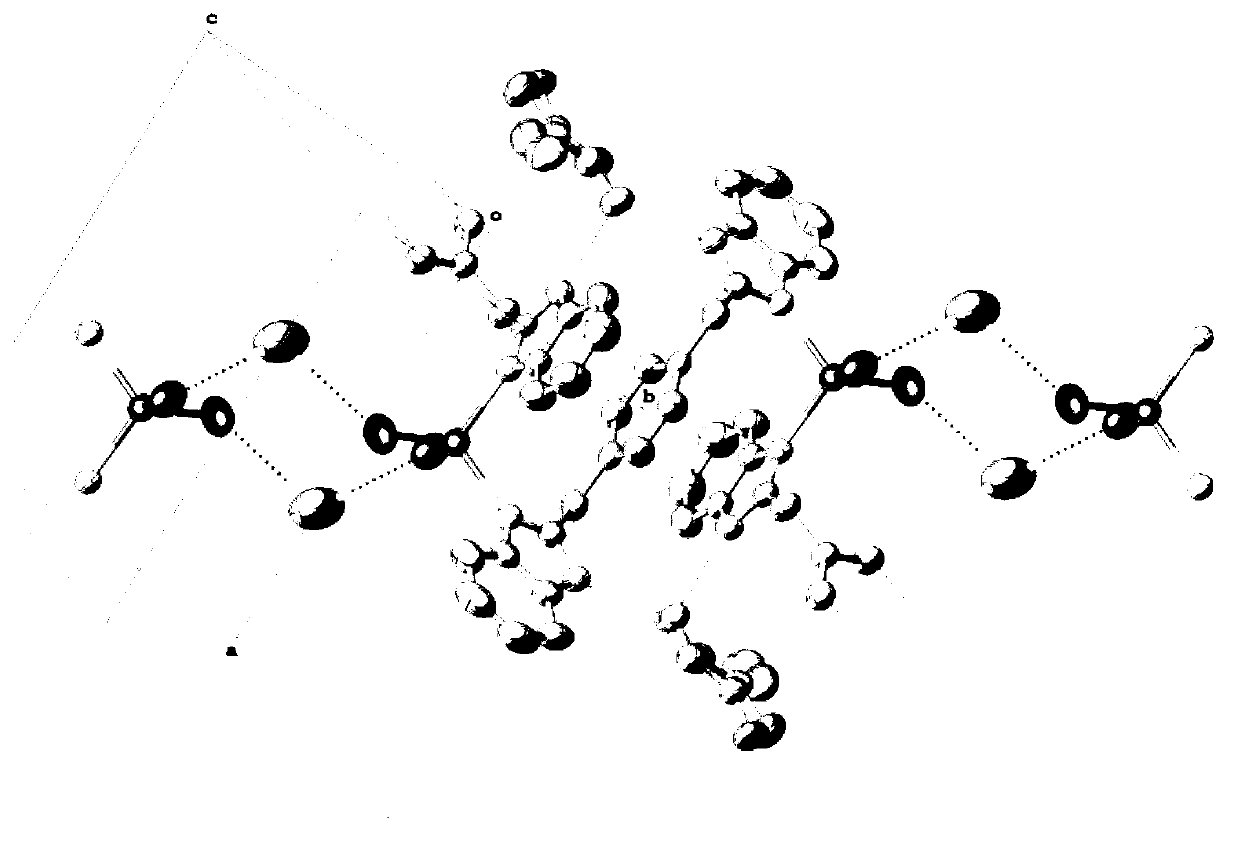
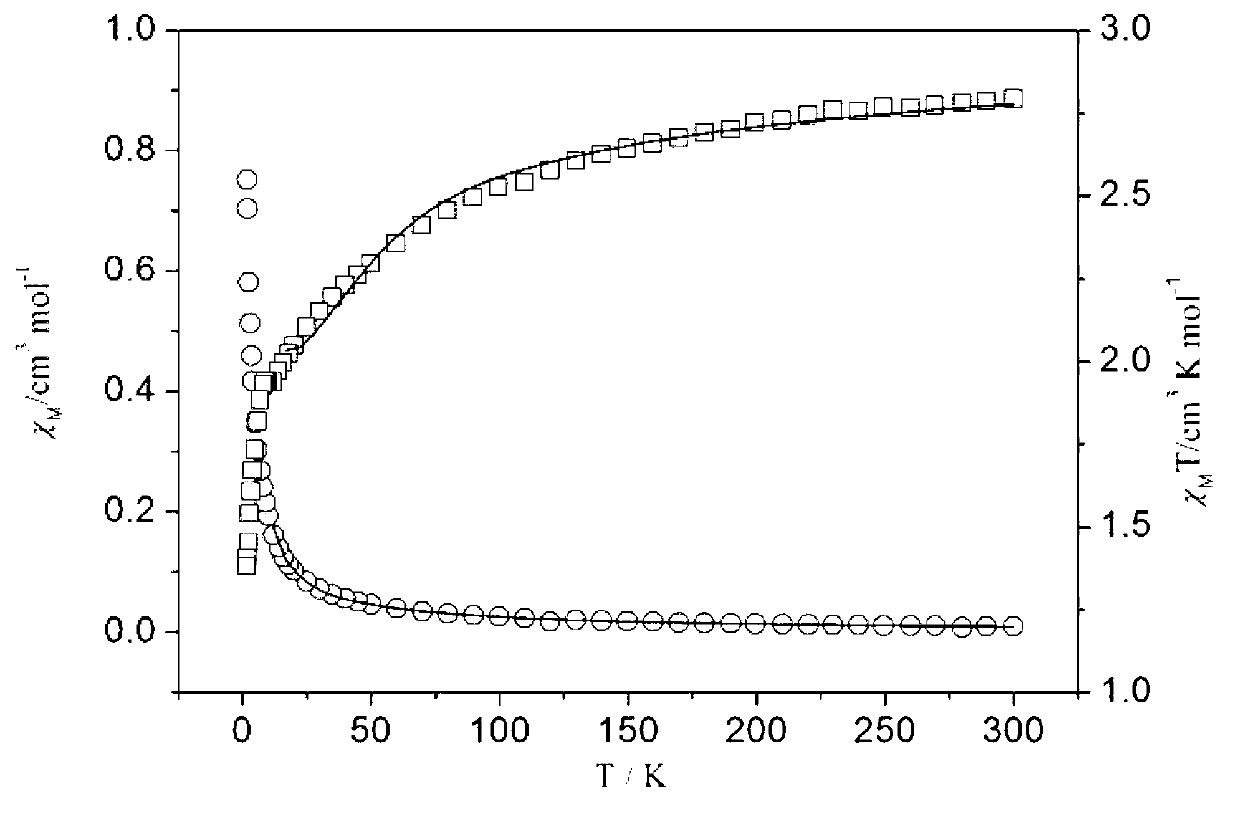
Nitrogenous multitooth transition metal complex and preparation method thereof
A transition metal, complex technology, applied in the direction of zinc organic compounds, cadmium organic compounds, cobalt organic compounds, etc., can solve the problem of no reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] with general formula C 28 h 32.64 N 6 o 2。32 Cl 2 The material composition represented by Co is taken as an example. In the general formula, M is cobalt element, m is 0.32, x is 0, and y is 1.
[0023] Its preparation method is as follows:
[0024] 1. Preparation of 2,2′-(1,4-dimethylbenzene-1′,1″-diyl)bisbenzimidazole ligand
[0025] Add 50mL polyphosphoric acid into the reactor, preheat to 100°C, stir, add 5.8g terephthalic acid and 7.2g m-phenylenediamine in sequence, the molar ratio of terephthalic acid to m-phenylenediamine is 1: 2. Stir and react at 200-220°C for 4-5 hours, cool to 70°C, add ice water 3 times the volume of the reactant to mix, continue to stir and react for 0.5 hour, filter with suction, wash with water, wash with saturated Na 2 CO 3 The pH value of the solution was adjusted to 9, suction filtered and washed with water to obtain a crude product, which was recrystallized from a solution with a volume ratio of dimethylformamide to water of 1:...
Embodiment 2
[0032] with general formula C 28 h 32.64 N 6 o 2。32 Cl 2 The material composition represented by Cd is taken as an example. In the general formula, M is cadmium element, m is 0.53, x is 0, and y is 1.
[0033] Its preparation method is as follows:
[0034] Preparation of 2,2′-(1,4-dimethylbenzene-1′,1″-diyl)bisbenzimidazole ligand Step 1 is the same as in Example 1.
[0035] In step 2 of preparing nitrogen-containing multidentate transition metal complexes, equimolar CdCl 2 4H 2 O instead of CoCl 2 ·6H 2 O, the other steps of this step are the same as in Example 1, and a nitrogen-containing multidentate transition metal complex is prepared with a yield of 65.0%.
[0036] The prepared nitrogen-containing multidentate transition metal complex belongs to the triclinic crystal system, the P-1 space group, and the unit cell parameter a is b for c for α is 71.759°, β is 88.915°, and γ is 77.546°.
[0037] Elemental analysis of the prepared product, theoretical value:...
Embodiment 3
[0040] with general formula C 28 h 32 N 8 o 8 The material composition represented by Zn is taken as an example. In the general formula, M is zinc element, m is 0, x is 1, and y is 0.
[0041] Its preparation method is as follows:
[0042] Preparation of 2,2′-(1,4-dimethylbenzene-1′,1″-diyl)bisbenzimidazole ligand Step 1 is the same as in Example 1.
[0043] In step 2 of preparing nitrogen-containing multidentate transition metal complexes, equimolar ZnNO 3 ·6H 2 O instead of CoCl 2 ·6H 2 O, the other steps of this step are the same as in Example 1, and a nitrogen-containing multidentate transition metal complex is prepared with a yield of 50.4%.
[0044] The prepared nitrogen-containing multidentate transition metal complex belongs to the triclinic crystal system, the P1 space group, and the unit cell parameter a is b for c for α is 74.7010°, β is 86.4200°, and γ is 73.3420°.
[0045] Elemental analysis of the prepared product, theoretical value: C 49.90%, H 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com