Monoimino pyrrole iron and preparation method and application thereof
A monoimine pyrrole, -CH3 technology, applied in the direction of iron organic compounds, etc., can solve the problems of transition metal complex catalysts that are rarely explored, and achieve good industrial application prospects, high yield, and less by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
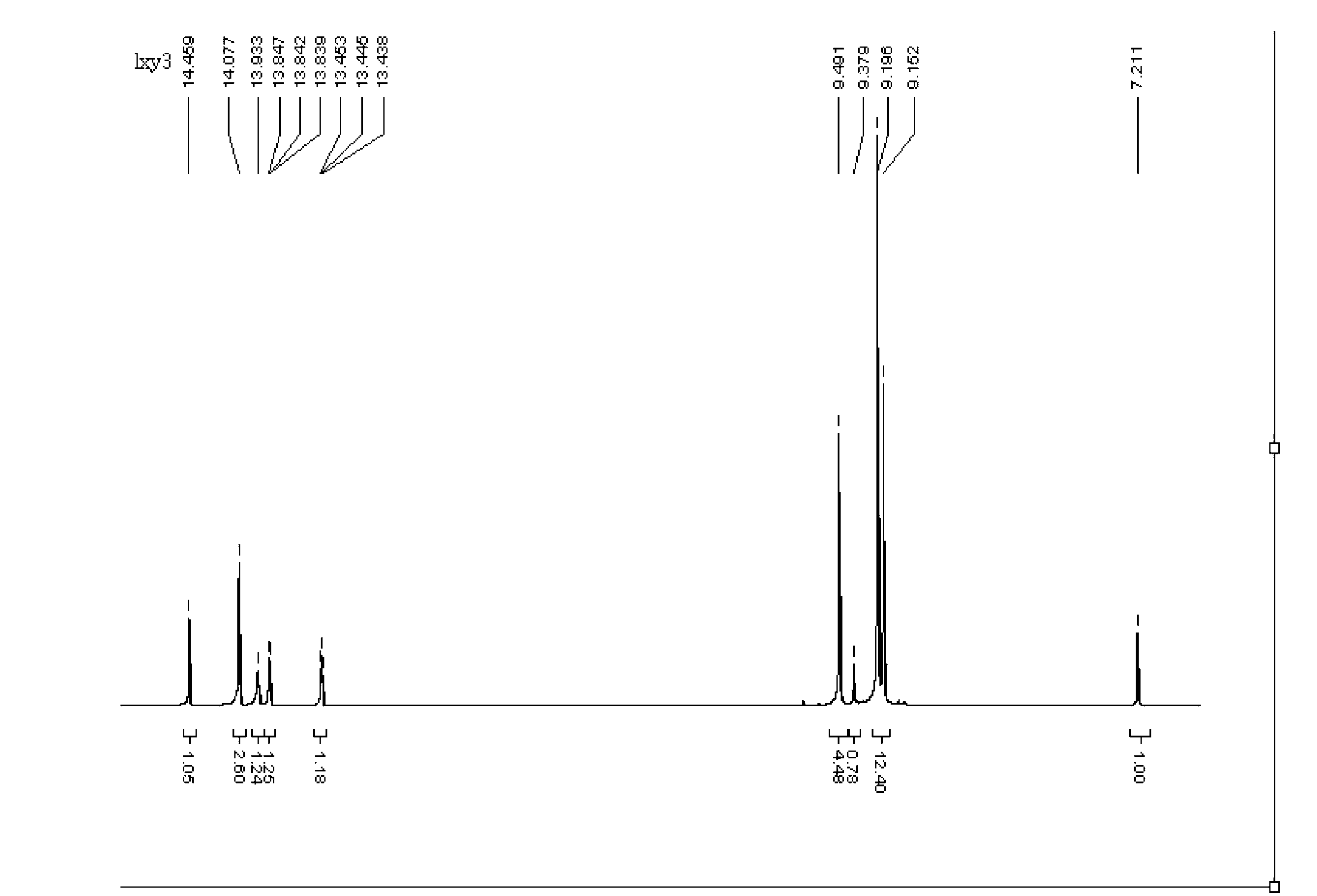
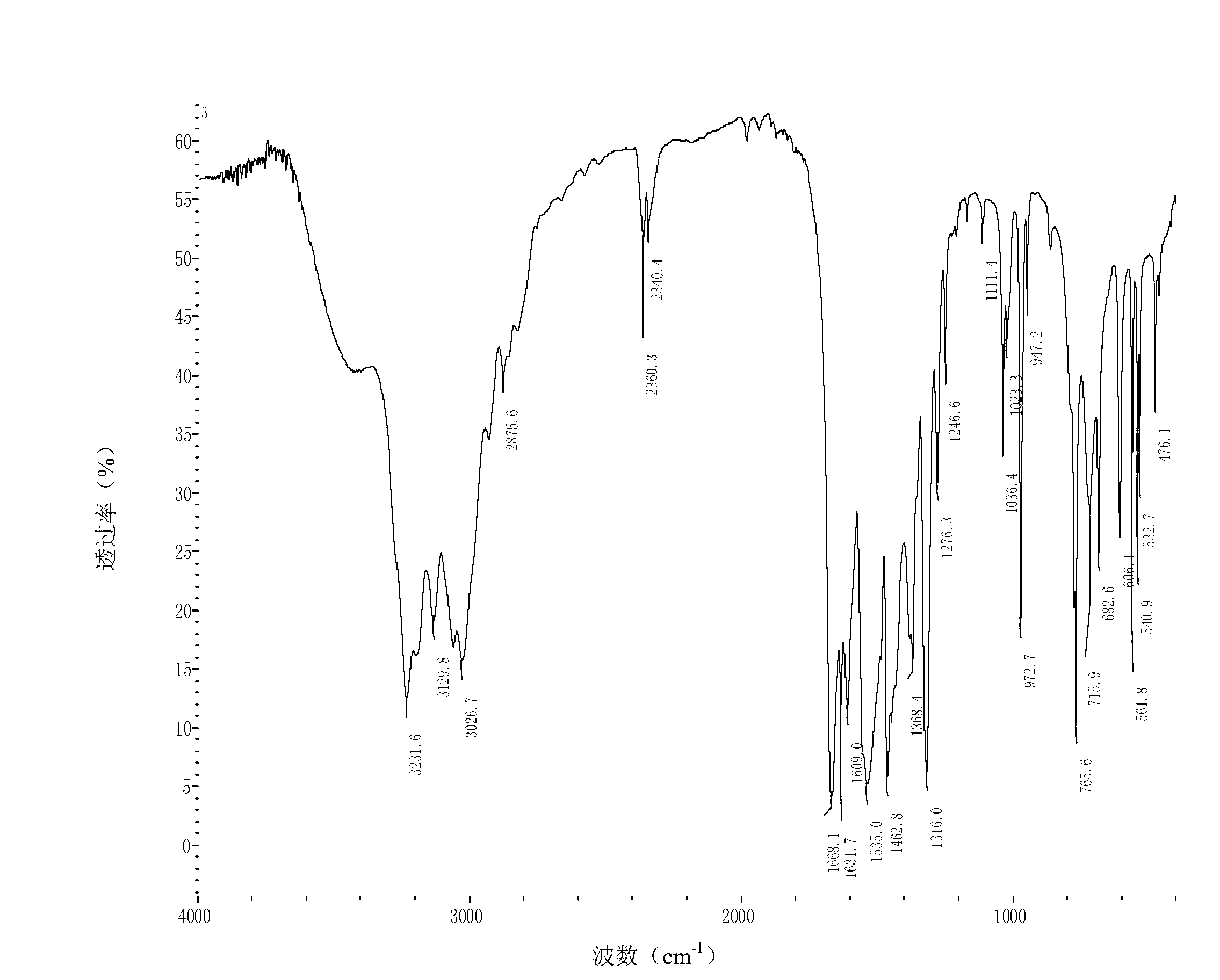
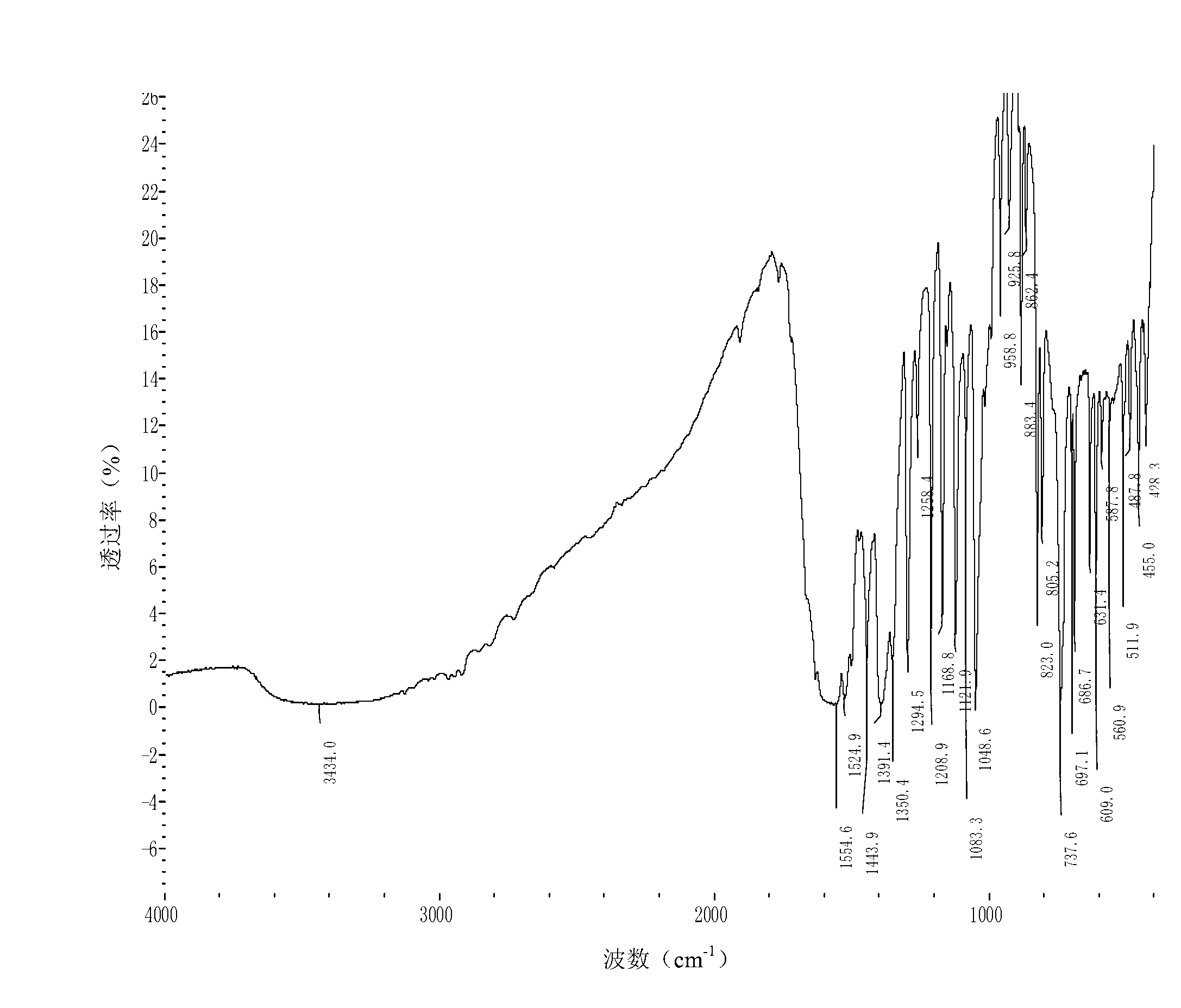
Image
Examples
Embodiment 1
[0026] The general structural formula of monoimine pyrrole iron is:
[0027] Formula I
[0028] where R 1 for -CH 3 ,R 2 is H, X is Cl - .
[0029] The chemical name is: dichloro 2-{1-[(2,6-xylyl)imine]ethyl}pyrrolate iron (II)
[0030] The preparation method of above-mentioned monoimine pyrrole iron is made up of the following steps:
[0031] (1) Mix 0.1313g of 2-acetylpyrrole and 0.1342g of substituted aniline of formula II according to the molar ratio of 1:1.2, and react under microwave conditions with a power of 700W for 25 minutes. The crude product is subjected to conventional chromatography Separation and purification give monoimine pyrrole, namely 2-{1-[(2,6-xylyl)imine]ethyl}pyrrole. R in Formula II 1 for -CH 3 ,R 2 for -H.
[0032] (2) Add 5mL of FeCl with a concentration of 20mmol / L 2 Ethanol solution and 4.5 mL of 2-{1-[(2,6-xylyl)imine] ethyl}pyrrole ethanol solution prepared in step (1) with a concentration of 20 mmol / L under nitrogen protection at ...
Embodiment 2
[0034] In the present embodiment, the general structural formula of monoimine pyrrole iron is formula I, wherein R 1 for -CH 3 ,R 2 is H, X is Cl - , the chemical name is: Dichloro·2-{1-[(2,6-xylyl)imine]ethyl}pyrrolate iron (II).
[0035] The preparation method of above-mentioned monoimine pyrrole iron is made up of the following steps:
[0036] (1) Mix 0.1313g of 2-acetylpyrrole with 0.1118g of substituted aniline of formula II according to the molar ratio of 1:1, react for 25 minutes under microwave conditions with a power of 700W, and chromatographically separate the crude product according to conventional methods , purified to obtain monoimine pyrrole;
[0037] where R in formula II 1 for -CH 3 , R 2 for -H.
[0038] (2) Add 5mL of FeCl with a concentration of 20mmol / L 2 The ethanol solution was mixed with 4 mL of monoimine pyrrole ethanol solution with a concentration of 20 mmol / L under nitrogen protection at a molar ratio of 1:0.8, reacted at room temperature for...
Embodiment 3
[0040] In the present embodiment, the general structural formula of monoimine pyrrole iron is formula I, wherein R 1 for -CH 3 , R 2 is H, X is Cl - , the chemical name is: Dichloro·2-{1-[(2,6-xylyl)imine]ethyl}pyrrolate iron (II).
[0041] The preparation method of above-mentioned monoimine pyrrole iron is made up of the following steps:
[0042] (1) Mix 0.1313g of 2-acetylpyrrole with 0.1677g of substituted aniline of formula II according to the ratio of molar ratio of 1:1.5, react for 25 minutes under microwave conditions with a power of 700W, and chromatographically separate the reaction crude product according to conventional methods, Purify to obtain monoimine pyrrole; wherein R in formula II 1 for -CH 3 , R 2 for -H.
[0043] (2) Add 5mL of FeCl with a concentration of 20mmol / L 2 Ethanol solution and 5 mL of monoimine pyrrole ethanol solution with a concentration of 20 mmol / L were mixed under nitrogen protection at a molar ratio of 1:1, reacted at room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com