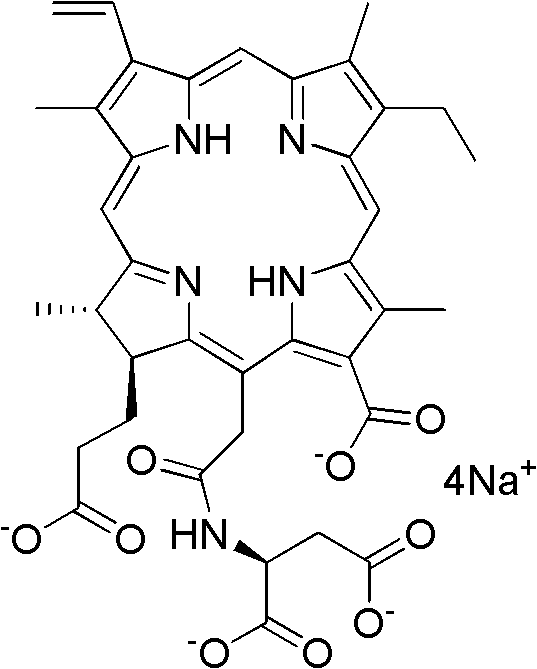
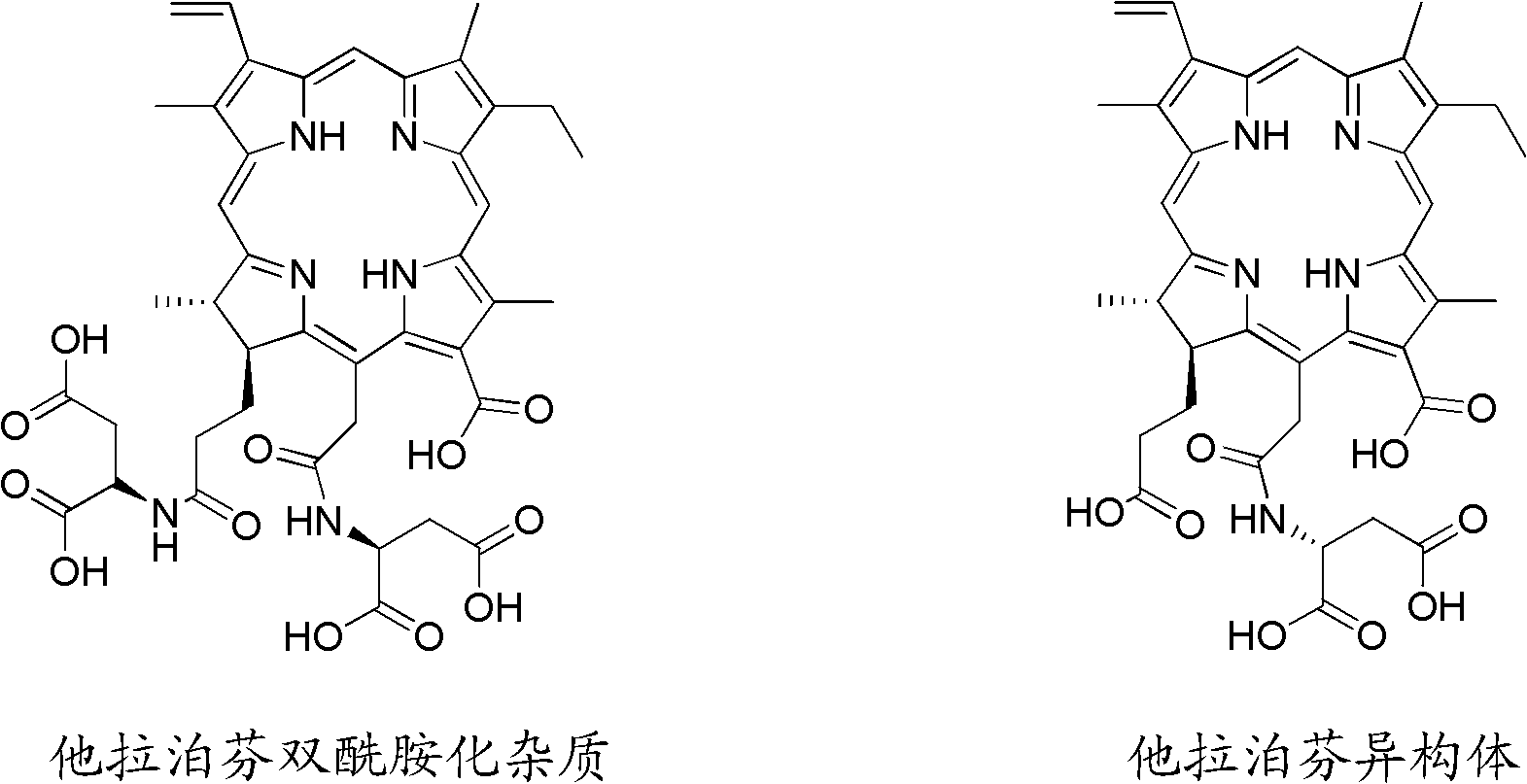
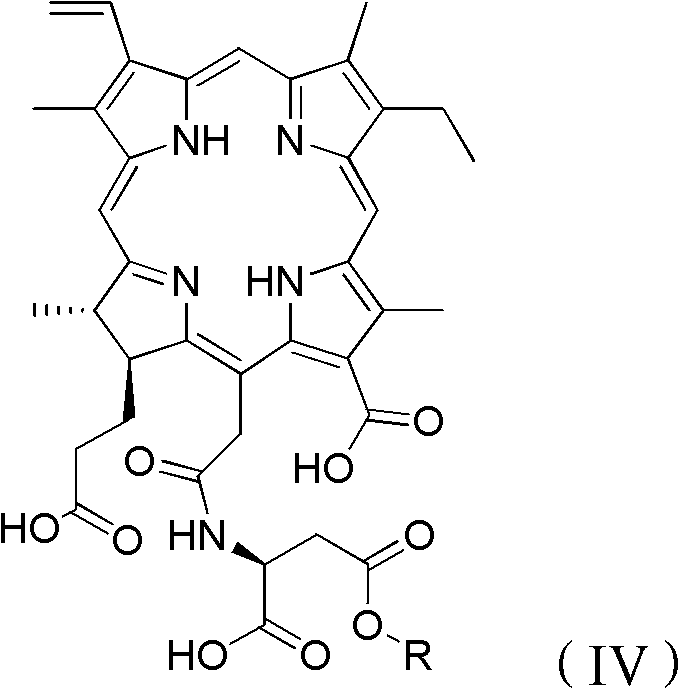
Preparation method for Talaporfin and intermediate thereof
A technology of taraporfin and its compounds, which is applied in the field of preparation of taraporfin and its intermediates, can solve problems such as difficult storage, unstable intermediates, and difficulties in industrial production, and achieves non-dangerous steps, improved selectivity, The effect of reducing the possibility of racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Preparation of chlorin e6 monoaspartic acid-4-tert-butyl ester:
[0025] 30g of chlorin e6 was suspended in dichloromethane, 9.6g of EDC and 2.4g of p-dimethylaminopyridine were added, and the reaction was carried out at room temperature. TLC detected that the reaction of the raw materials was basically completed, and 11.4g of L-aspartic acid-4-tert. Butyl ester, TLC detection intermediate acid anhydride reaction is completed, stop reaction, medium pressure preparative chromatographic separation (separation condition: 80mm × 400mm stainless steel column, 300-400 purpose silica gel, dichloromethane: methanol: glacial acetic acid is 25: 1: 0.1 is eluent), collect the target spots, evaporate the solvent to dryness to obtain 25.9 g of chlorin e6 monoaspartate-4-tert-butyl ester, with a yield of 67.1%. By HPLC analysis, the purity was 99.09%.
[0026] MS: 768.6 (M+1) + , 790.6(M+Na) + .
[0027] 1 HNMR: (DMSO, 600M): 9.79(1H,S), 9.73(1H,S), 9.16(1H,S), 8.32(1H,m), 8...
Embodiment 2
[0034] (1) Preparation of chlorin e6 monoaspartic acid-4-tert-butyl ester:
[0035] Suspend 30g of chlorin e6 in chloroform, add 10.4g of DCC and 2.4g of p-dimethylaminopyridine, react at -20°C, TLC detects that the reaction of the raw materials is basically completed, add 7.6g of L-aspartic acid-4 -tert-butyl ester, the completion of the reaction of the intermediate acid anhydride monitored by TLC, the reaction was stopped, and the medium pressure preparative chromatographic separation (separation conditions: 80mm × 400mm stainless steel column, 300-400 mesh silica gel, dichloromethane: methanol: glacial acetic acid was 25: 1: 0.1 is the eluent), collect the target points, evaporate the solvent to obtain 27.1 g of chlorin e6 monoaspartate-4-tert-butyl ester, and the yield is 70.2%. By HPLC analysis, the purity was 99.04%.
[0036] MS: 768.6 (M+1) + , 790.6(M+Na) + .
[0037] 1 HNMR: (DMSO, 600M): 9.79(1H,S), 9.73(1H,S), 9.16(1H,S), 8.32(1H,m), 8.10(1H,dd), 6.48(1H,dd), ...
Embodiment 3
[0045] (1) Preparation of chlorin e6 monoaspartic acid-4-trimethylsilyl ester:
[0046] 3g of chlorin e6 was suspended in dichloromethane, 1.36g of EDC and 0.24g of p-dimethylaminopyridine were added, and the reaction was carried out at 80 °C. Trimethylsilyl ester, TLC monitoring intermediate acid anhydride reaction is completed, stop reaction, medium pressure preparative chromatographic separation (separation conditions: 40mm × 400mm stainless steel column, 300-400 mesh silica gel, dichloromethane: methanol: glacial acetic acid is 25: 1: 0.1 is the eluent), collect the target points, evaporate the solvent to obtain chlorin e6 monoaspartic acid-4-trimethylsilyl ester 2.5 g, the yield is 63.3%, and the purity is 99.08%.
[0047] MS: 785.4 (M+1) + , 817.5(M+Na) + .
[0048] (2) Preparation of talaporfin:
[0049] Dissolve 20 g of chlorin e6 monoaspartate-4-tert-butyl ester in 2000 ml of 1 mol / L trifluoroacetic acid methanol solution at room temperature, and react at 0° C. un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com