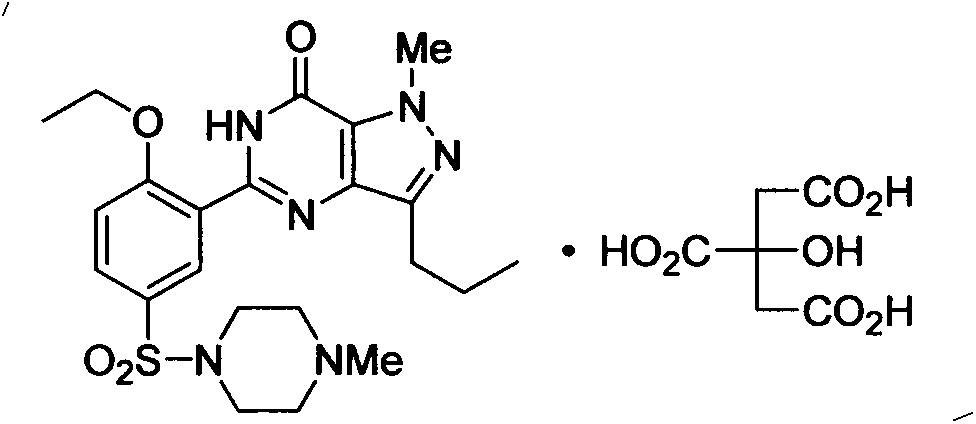
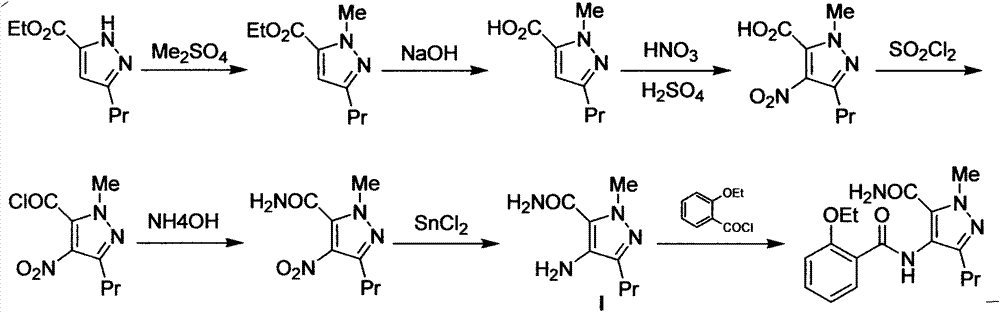
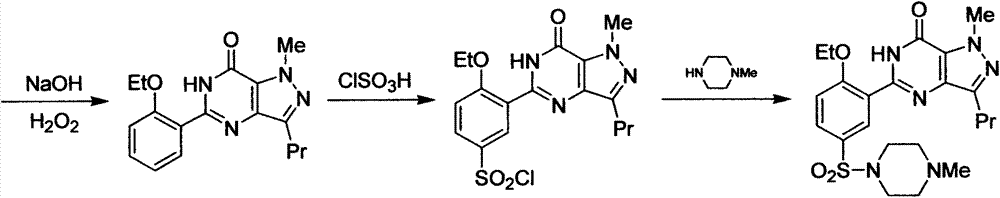
New green synthesis process of sildenafil intermediate compound 4-amino-1-methyl-3-n-propyl pyrazole-5-formamide
A technology of n-propylpyrazole and formamide, which is applied in the field of preparation of drugs for treating male erectile dysfunction and drug intermediates, can solve the problems of unsatisfactory separation and purification, and achieve easier control of reactions, less heat release, The effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 4-nitro-1-methyl-3-n-propylpyrazole-5-carboxamide (III)
[0028] Add 16.7 g of 1-methyl-3-n-propylpyrazole-5-carboxamide into 50 mL of dichloromethane, control the temperature below 15° C., and add fuming nitric acid (12 g) dropwise. Stir the reaction at 20-25° C. and track it by TLC until the raw material point disappears. Then the reaction solution was poured into ice water, stirred, separated, the organic phase was washed with water (20mL×2), dried, filtered, and concentrated dichloromethane to dryness to obtain 4-nitro-1-methyl-3-n-propyl Pyrazole-5-carboxamide 19.3g, yield 91%, HPLC purity 95.6%.
Embodiment 2
[0029] Example 2: Preparation of 4-nitro-1-methyl-3-n-propylpyrazole-5-carboxamide (III)
[0030] 16.7g of 1-methyl-3-n-propylpyrazole-5-carboxamide was added to 50mL of dichloromethane, the temperature was controlled below 15°C, 25g of concentrated nitric acid was added dropwise, and after stirring for 40 minutes, the temperature was controlled at 5g of concentrated sulfuric acid was added dropwise below 15°C. Stir the reaction at 20-25° C. and track it by TLC until the raw material point disappears. Then the reaction solution was poured into ice water, stirred, separated, the organic phase was washed with water (20mL×2), dried, filtered, and concentrated dichloromethane to dryness to obtain 4-nitro-1-methyl-3-n-propyl Pyrazole-5-carboxamide 20.1 g, yield 95%, HPLC purity 96.8%.
Embodiment 3
[0031] Embodiment 3: the preparation of 4-amino-1-methyl-3-n-propylpyrazole-5-carboxamide (I)
[0032] 21.2g of 4-nitro-1-methyl-3-n-propylpyrazole-5-carboxamide, [bmim][BF 4 ] 100mL, 13g of zinc particles, 10mL of water and 9.5g of ammonium formate were mixed, stirred rapidly at room temperature, followed by TLC until the raw materials disappeared. Add diethyl ether for extraction (150mL×3), combine the diethyl ether phases, and concentrate to dryness to obtain 16.2 g of 4-amino-1-methyl-3-n-propylpyrazole-5-carboxamide, with a yield of 89%, HPLC purity was 95.1%.
[0033] The ionic liquid layer was dissolved in 100 mL of dichloromethane, dried by adding anhydrous sodium sulfate, filtered off the desiccant, and concentrated to dryness under reduced pressure to obtain 92 mL of reusable ionic liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com