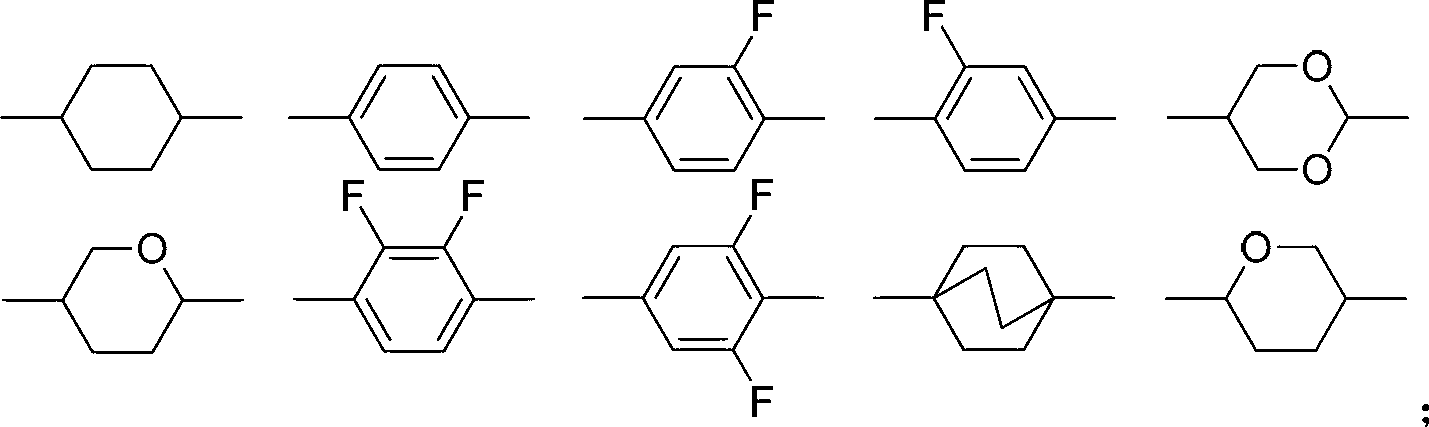
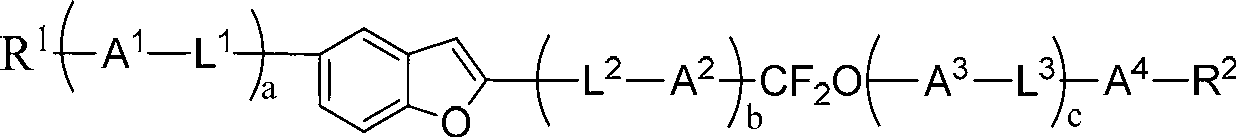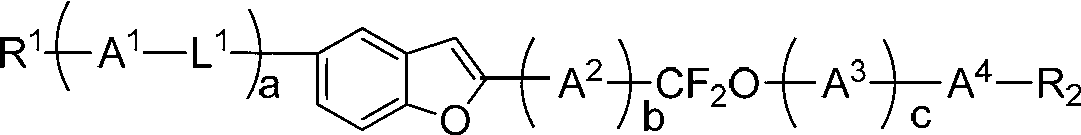Liquid crystal compound containing benzoxazole and difluoromethylenedioxy bridged linkage and preparation method and application thereof
A compound and liquid crystal technology, applied in chemical instruments and methods, organic chemistry, liquid crystal materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
[0066] step 1:
[0067]
[0068] Add 0.1mol 4-propylbenzofuran (reactant) and 80ml tetrahydrofuran (solvent) to the reaction flask, protect it with nitrogen, cool down to -10°C, add 0.1mol n-butyllithium (reactant) petroleum ether dropwise (Solvent) solution, the dropwise addition is completed within 1 hour, and the negative ionization reaction is carried out with stirring at 0°C for 30 minutes. Then cool down to -30°C, add dropwise 0.11mol iodine (reactant) in 70ml tetrahydrofuran (solvent) solution within 1 hour. Sodium hydrogen aqueous solution (reducing agent) to remove excess iodine in the reaction, add water to wash, extract petroleum ether (solvent) and separate liquids, wash the organic phase with water until neutral, evaporate the solvent to dryness, distill under reduced pressure, collect 132 ℃ ~ 134 ℃ / 2mmHg fraction to obtain compound (1-a) with a yield of 85% and a purity of 97% by gas chromatography.
[0069] Step 2:
[0070]
[0071] Add 0.0...
Embodiment 2
[0088] According to the 4-step reaction of Example 1, the 3,5-difluorophenylboronic acid in step 2 is replaced by 2-fluoro-3', 5'-difluorobiphenylboronic acid, and the products are fed and reacted sequentially downwards. In step 4 The following compounds were prepared by substituting 3,4,5-trifluorophenol for 3,5-difluoro-4-trifluoromethylphenol.
[0089]
[0090] Wherein, the intermediates of each step are respectively
[0091] step 1
[0092]
[0093] step 2
[0094]
[0095] step 3
[0096]
[0097] step 4
[0098]
[0099] The experimental results are as follows:
[0100] 1. GC: 99.8%
[0101] 2. MS: m / z%189(6.77)262(3.91)386(9.44)468(100)612(M + 0.56)
[0102] 3. MP: 146.5°C
[0103] As can be seen from the above, the product has a correct structure and is a compound shown in formula I12. The test results of its liquid crystal performance are as follows:
[0104] 4. △ε: 21.3 (20°C, 1000Hz)
[0105] 5. △n: 0.22 (20°C, 589nm)
[0106] 6. CP: 148°C ...
Embodiment 3~44
[0108] With reference to the method of Example 1 and 2, only the reactant substituent is replaced according to the corresponding product, and the compound shown in the following formula I is obtained:
[0109]
[0110]
[0111]
[0112]
[0113]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com