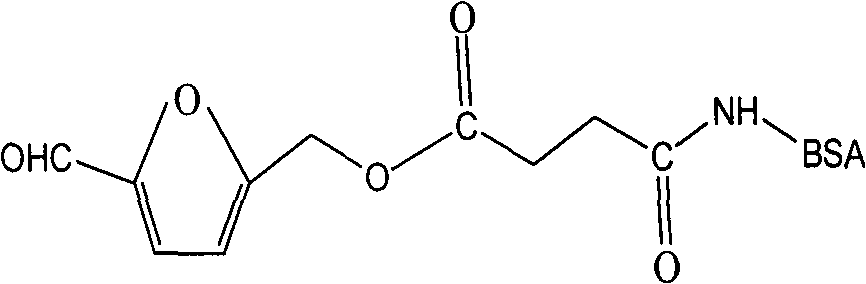
Antibody against 5-hydroxymethyl furfural
A hydroxymethylfurfural and antibody technology, applied in the biological field, can solve the problems of high cost, complicated and complicated operation, slow analysis speed and the like, and achieve the effects of few reaction steps, simple synthesis method and easy acquisition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
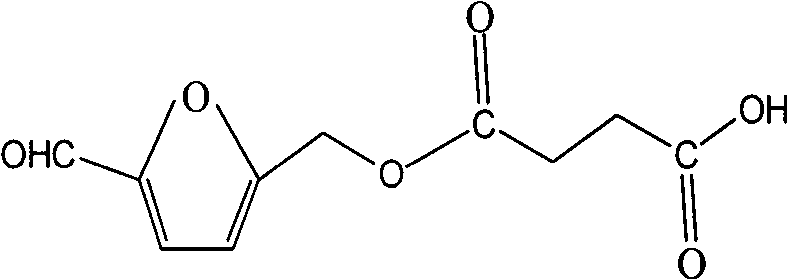
[0033] 1. Hapten Synthesis
[0034] Hapten molecular weight of the present invention is 226, and molecular structural formula is:
[0035]
[0036] The specific preparation method is: add 144.9mg (1.15mmol) of 5-hydroxymethylfurfural into a small amount of newly prepared tetrahydrofuran solution to dissolve it, then add 140.3mg (1.15mmol) of 4-lutidine, triethylamine (2.3 mmol) 0.317ml, succinic anhydride (2.3mmol) 230mg, stirred and reacted overnight (about 9 hours) in an oil bath at 60°C. The solvent was removed under reduced pressure, and the residue was dissolved with methanol / dichloromethane / glacial acetic acid (1:9.5:0.05 V / V / V). The primary product was purified by column chromatography with methanol / dichloromethane / glacial acetic acid (1:9.5:0.05 V / V / V). The obtained product was collected and evaporated under vacuum to obtain a relatively pure light yellow hapten.
[0037] Use 10g of 200-300 mesh silica gel for packing the column, and add 1.0g of the same specific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com