Gemcitabine hydrochloride for injection as well as preparation method thereof
A technology for gemcitabine hydrochloride and injection, which is applied in the field of gemcitabine hydrochloride for injection and its preparation, which can solve the problems of introducing allergens and the safety of preparations cannot be guaranteed, so as to increase stability, avoid water degradation, and improve product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
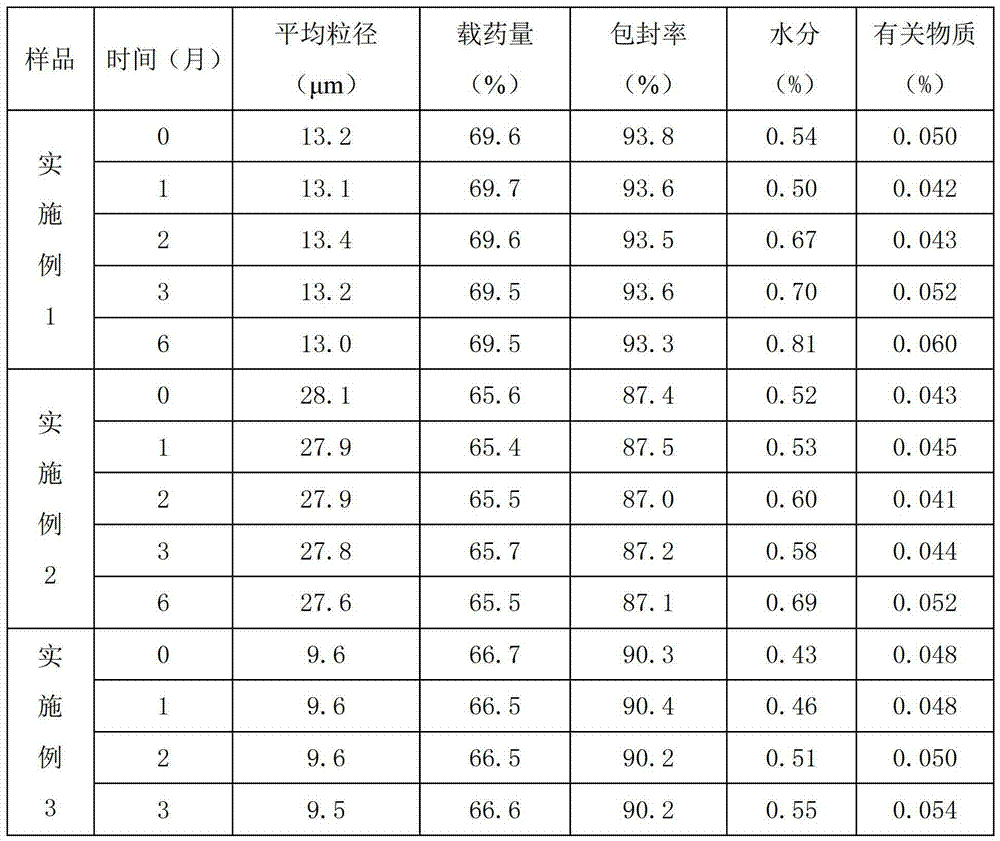
[0030] Dissolve 1kg of gemcitabine hydrochloride and 1.6kg of gelatin in 25L of water, dissolve and mix well as the inner water phase (phase W1); 75 / 25, molecular weight 6000) was dissolved in 180L dichloromethane as the oil phase (O phase); 22kg polyvinyl alcohol was dissolved in water for injection at 80°C, so that the mass volume concentration of polyvinyl alcohol was 1.0% (w / v), as the external water phase (W2 phase); slowly drop the W1 phase into the O phase, emulsify at a high speed of 18000r / min for about 4min, cool to 10°C, use it as colostrum, and add it to the W2 phase, 15000r / min high-speed oscillating emulsification for about 5 minutes, 5000r / min for 3-5h to evaporate dichloromethane, 10000r / min high-speed centrifugation, use water for injection to wash 3-5 times to remove polyvinyl alcohol, sub-package, half stopper, sample into the box, Freeze drying is carried out as follows:
[0031] (1) After putting the product into the freeze dryer, first lower the temper...
Embodiment 2
[0035] Dissolve 1kg of gemcitabine hydrochloride and 1.8kg of gelatin in 30L of water, dissolve and mix well as the inner water phase (W1 phase); 75 / 25, molecular weight 6000) was dissolved in 200L dichloromethane as the oil phase (O phase); 25kg polyvinyl alcohol was dissolved in water for injection at 80°C, so that the mass volume concentration of polyvinyl alcohol was 0.98% (w / v), as the external water phase (W2 phase); slowly drop the W1 phase into the O phase, emulsify at a high speed of 15000r / min for about 5min, cool to 10°C, use it as colostrum, and add it to the W2 phase, 15000r / min high-speed oscillating emulsification for about 5 minutes, 5000r / min for 3-5h to evaporate dichloromethane, 15000r / min high-speed centrifugation, use water for injection to wash 4-7 times to remove polyvinyl alcohol, sub-package, half stopper, sample into the box, Freeze-drying was carried out according to the steps of Example 1.
Embodiment 3
[0037]Dissolve 1kg gemcitabine hydrochloride and 1kg gelatin in 20L water, dissolve and mix well as the inner water phase (W1 phase); mix 15kg polylactic acid-glycolic acid (the ratio of lactide to glycolide in polylactic acid-glycolic acid is 75 / 25, molecular weight 6000) was dissolved in 100L dichloromethane as the oil phase (O phase); 16kg polyvinyl alcohol was dissolved in water for injection at 80°C, so that the mass volume concentration of polyvinyl alcohol was 1.05% (w / v ), as the external water phase (W2 phase); slowly drop the W1 phase into the O phase, emulsify at a high speed of 15000r / min for about 5min, cool to 10°C, and add it to the W2 phase, 20000r / min Min high-speed oscillation emulsification for about 3 minutes, 4000r / min for 3-5h to evaporate dichloromethane, 10000r / min high-speed centrifugation, use water for injection to wash 3-5 times to remove polyvinyl alcohol, subpackage, half stopper, sample into the box, press The steps of Example 1 were freeze-drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com