Immunity enhancing agent, inactivated vaccine, and preparation method thereof
An immune enhancer and inactivated vaccine technology, applied in the field of biopharmaceuticals, can solve the problems of large side effects and external stimulation of pigs, and achieve the effects of low cost, enhanced immune effect, and short immune window period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
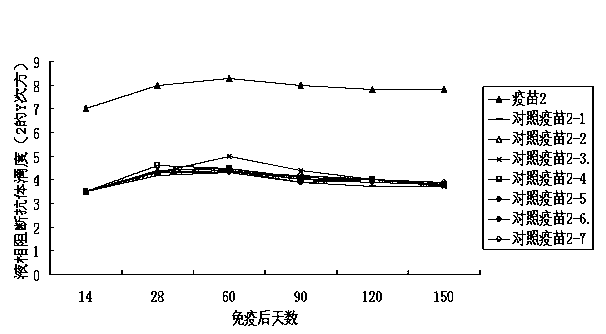
[0037] Example 1 Influence of immune enhancer 1 on the immune efficacy of inactivated foot-and-mouth disease vaccine
[0038] 1. Experimental materials
[0039] The inactivated porcine type O foot-and-mouth disease virus liquid (the strain is 98 strains of porcine type O foot-and-mouth disease virus from Myanmar) was inactivated by diethyleneimine, and the content of 146s was 4ug / mL.
[0040] Healthy susceptible piglets aged 6-7 weeks, liquid-phase blocking ELISA antibody titer ≤ 1:8.
[0041] 2. Preparation of vaccine:
[0042] Immunopotentiator 1 contains 2 mg / mL MPL, 20 mg / mL MDP, and 0.4 mg / mL β-glucan, and the solvent is distilled water.
[0043] An aqueous phase solution was prepared by mixing the immune enhancer 1 with the inactivated porcine O-type foot-and-mouth disease virus liquid in a volume ratio of 1:3. The ISA206 and the aqueous solution were first placed at room temperature for about 30 minutes. Put ISA206 into the emulsification tank, put the aqueous solut...
Embodiment 2
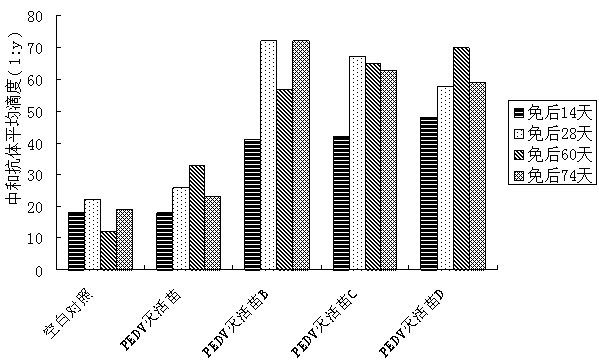
[0058] Example 2 The effect of immune enhancer 2 on the immune efficacy of inactivated foot-and-mouth disease vaccine
[0059] 1. Experimental materials
[0060] The inactivated O-type swine foot-and-mouth disease virus liquid (the strain is 98 strains of porcine O-type FMD virus from Myanmar) was inactivated by diethyleneimine, and the content of 146s was 5.87ug / mL.
[0061] Healthy susceptible piglets aged 6-7 weeks, liquid-phase blocking ELISA antibody titer ≤ 1:8.
[0062] 2. Preparation of vaccine:
[0063] Formulated immune enhancer 2. Immunopotentiator 2 contains 2 mg / mL MPL, 40 mg / mL MDP, and 0.4 mg / mL β-glucan, and the solvent is distilled water.
[0064] The immune enhancer 2 is mixed with the inactivated swine foot-and-mouth disease virus liquid in a volume ratio of 1:1 to obtain an aqueous phase solution. The ISA206 and the aqueous solution were first placed at room temperature for about 30 minutes. Put ISA206 into the emulsification tank, put the aqueous solu...
Embodiment 3
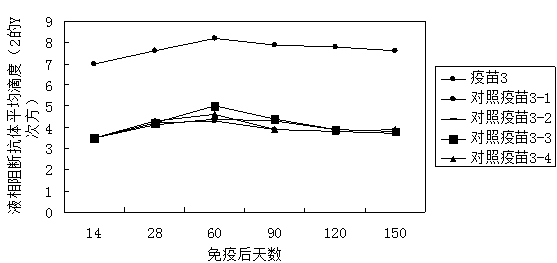
[0079] Example 3 Immunopotentiator 3 Influence on the immune efficacy of inactivated foot-and-mouth disease vaccine
[0080] 1. Experimental materials
[0081] The inactivated porcine type O foot-and-mouth disease virus liquid (the strain is 98 strains of porcine type O foot-and-mouth disease virus from Myanmar) was inactivated by diethyleneimine, and the content of 146s was 5.87ug / mL.
[0082] Healthy susceptible piglets aged 6-7 weeks were tested with a liquid-phase blocking ELISA kit for foot-and-mouth disease (Lanzhou Veterinary Research Institute) to obtain a liquid-phase blocking ELISA antibody titer of ≤1:8.
[0083] 2. Preparation of vaccine:
[0084] Immunopotentiator 3 contains 0.1 mg / mL MPL, 2 mg / mL MDP, and 0.1 mg / mL β-glucan in distilled water.
[0085] The immune enhancer 3 is mixed with the inactivated swine foot-and-mouth disease virus liquid in a volume ratio of 1:1 to obtain an aqueous solution. The ISA206 and the aqueous solution were first placed at ro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com