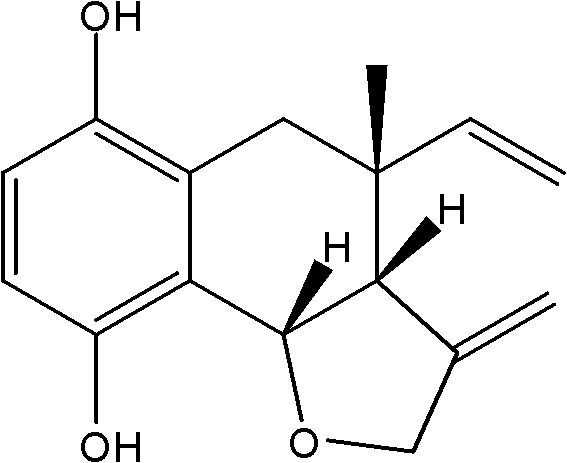
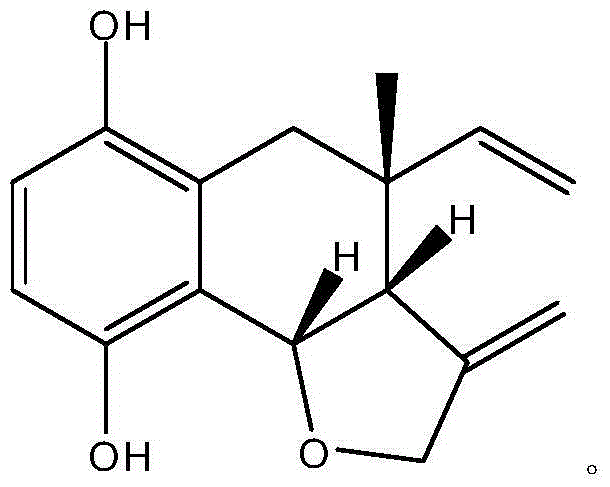
Boraginaceae phenol compound and purpose thereof in preparing anticomplement medicines
A technology of shikonol and compounds, applied in the field of traditional Chinese medicine pharmaceuticals, can solve problems such as the inhibitory effect of the complement system that has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of shikonbutyrin (arnebiol D)
[0016] 20kg of dry root of Xinjiang comfrey, the coarse powder was repeatedly cold soaked and percolated with 95% ethanol at room temperature and extracted several times, and the solvent was recovered under reduced pressure to obtain 820g of extract, which was suspended in distilled water and mixed with petroleum ether and ethyl acetate. Ester and n-butanol extraction to obtain 420 g of ethyl acetate extract. Take 180g of the ethyl acetate extract, go through silica gel column chromatography, and use petroleum ether (60-90°C)-acetone gradient elution to obtain 7 fractions Fr.1~Fr.7, Fr.2 go through silica gel column Ether-chloroform (5:1) was used as the eluent to obtain 10 fractions Fr.2-1~Fr.2-10, and the obtained fraction Fr.2-6 was recrystallized by petroleum ether-acetone (4:1) Compound JAE-4 (15 mg) was obtained, which was identified as a shikonol compound with a new structure by spectroscopy and named as arne...
Embodiment 2
[0017] Example 2 Anti-complement classical pathway test in vitro
[0018] Take 0.1ml of complement (guinea pig serum), add BBS to prepare a 1:5 solution, and dilute it to 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:320 with BBS 640 solution. Dissolve 1:1000 hemolysin, 0.1ml of each concentration of complement and 2% SRBC in 0.3ml BBS, mix well, put in a low-temperature high-speed centrifuge after 30min in a 37°C water bath, and centrifuge at 5000rpm and 4°C for 10min. Take 0.2ml of the supernatant from each tube and place it in a 96-well plate, and measure the absorbance at 405nm. A full hemolysis group (0.1ml 2% SRBC dissolved in 0.5ml triple distilled water) was also set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Taking the dilution of complement as the X-axis, the percentage of hemolysis caused by each dilution of complement is plotted as the Y-axis. ...
Embodiment 3
[0019] Example 3 Anti-complement alternative pathway test in vitro
[0020] Take 0.2ml of complement (human serum), add AP diluent to prepare a 1:5 dilution solution, and double-dilute to 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640 solution. Take 0.15ml of complement of each concentration, 0.15ml of AP diluent and 0.20ml of 0.5% RE, mix well, place in a low-temperature high-speed centrifuge after 30 minutes in a 37°C water bath, and centrifuge at 5000rpm and 4°C for 10 minutes. Take 0.2ml of the supernatant from each tube and place it in a 96-well plate, and measure the absorbance at 405nm. At the same time, a complete hemolysis group (0.20ml 0.5% RE dissolved in 0.3ml triple distilled water) was set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Taking the dilution of complement as the X-axis, the percentage of hemolysis caused by each dilution of compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com