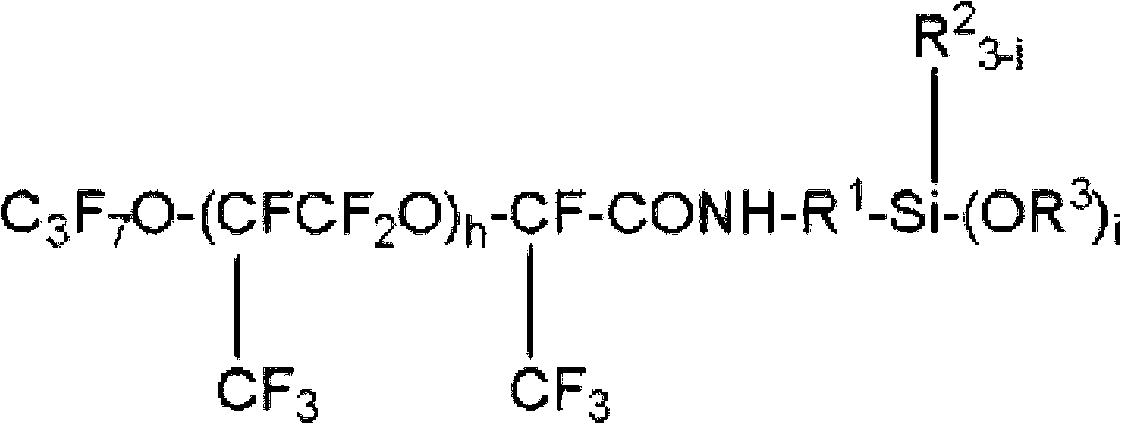
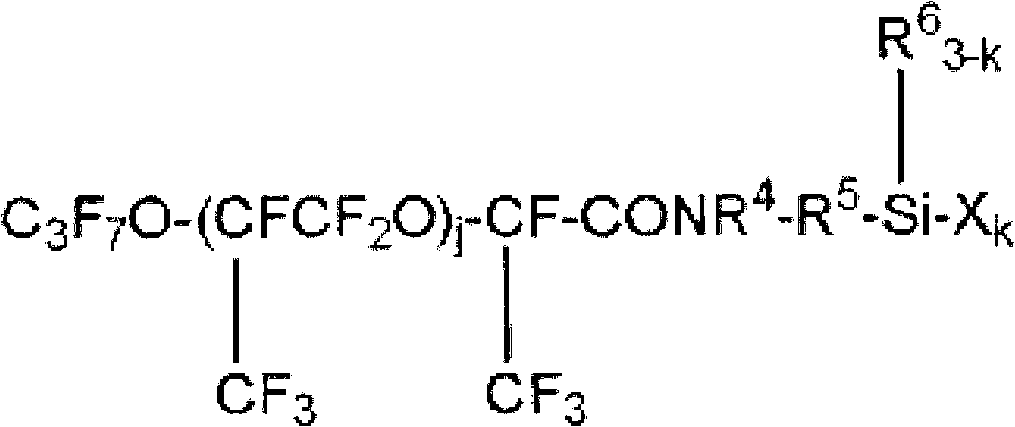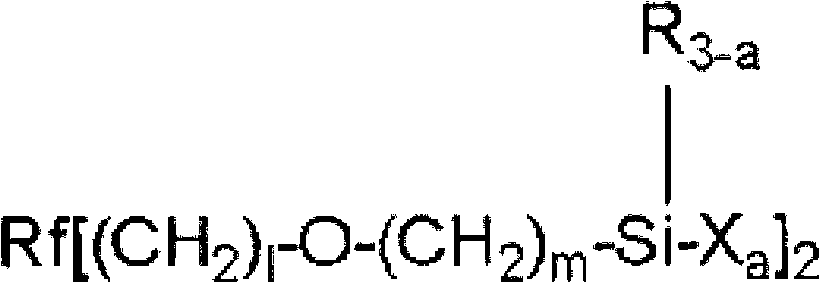Fluorooxyalkylene group-containing polymer composition, surface treatment agent containing the composition, and article and optical article treated with the surface treatment agent
An oxyalkylene-based and fluorooxyalkylene technology, which is applied in the directions of paints, optics and optical components containing biocides, can solve the problems of poor alkali resistance and reduced adhesion of substrates, and achieve excellent alkali resistance and substrates. The effect of excellent adhesion and low dynamic friction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0147] Into the reaction vessel, a mixture comprising (6a) 60%, (6b) 2% and (6c) 38% represented by the above formula (terminal-CF 3(68% of the total terminal groups in the mixture) 75g, 50g of 1,3 trifluorotoluene and 15g of tetrahydrofuran are dissolved in a mixed solvent, and bis(2-methoxyethoxy)sodium aluminum hydride is added dropwise 40% toluene solution 38g. After stirring at room temperature for 3 hours, an appropriate amount of hydrochloric acid was added, stirred well, and washed with water. Furthermore, the lower layer was taken out, and after distilling off the solvent, a part of the product mixture [mainly (6c) component] was further distilled off under reduced pressure to obtain 40 g of a liquid product. use 19 F-NMR, confirmed that in the mixture of the obtained products, the end-CF 3 The ratio of the group to the total terminal group was 65%, and it was confirmed that it contained a mixture of the following (7a), (7b) and (7c).
[0148] f 3 C(OC 2 f 4 ) ...
Embodiment 2)
[0165] 40g of the mixture containing (8a), (8b) and (8c), 140g of 1,3 trifluorotoluene, 20g of 1,4-bis(dimethylsilyl)benzene, chloroplatinic acid / vinylsiloxane 0.01g toluene solution of alkane complex (containing 2.5×10 Pt monomer -9 mol) were mixed and aged at 80°C for 3 hours. After the solvent was distilled off under reduced pressure, the separated polymer layer (lower layer) was recovered, washed with acetone, and then concentrated under reduced pressure to obtain 30 g of a liquid product. The obtained product was a mixture containing (11a), (11b) and (11c) represented by the following formula.
[0166]
[0167] 25g of the mixture containing the above (11a), (11b) and (11c), 25g of 1,3 trifluorotoluene, 1.1g of vinyltrimethoxysilane (VMS), chloroplatinic acid / vinylsiloxane complex 0.01 g of toluene solution (containing Pt monomer 2.5 x 10 -9 mol) were mixed and aged at 80°C for 3 hours. Then, the solvent and unreacted substances were distilled off under reduced pres...
Embodiment 1
[0173] About Example 1, except having used 1.1 g of octenyltrimethoxysilanes instead of 0.7 g of alkenyltrimethoxysilanes, it synthesize|combined by operation similar to Example 1. The resulting product is a mixture comprising (13a), (13b) and (13c) represented by the following formula, terminal-CF 3 The proportion of the bases relative to the total end groups in the mixture was 65% (composition 3).
[0174]
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com