Propylene glycol marinate sulfate-containing sustained-release preparation and preparation method thereof
A technology for sustained-release preparations and glycoside esters, which is applied in the directions of medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulations, etc. Balanced dosing and other issues to achieve the effect of small fluctuation of blood drug concentration, stable release and balanced release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
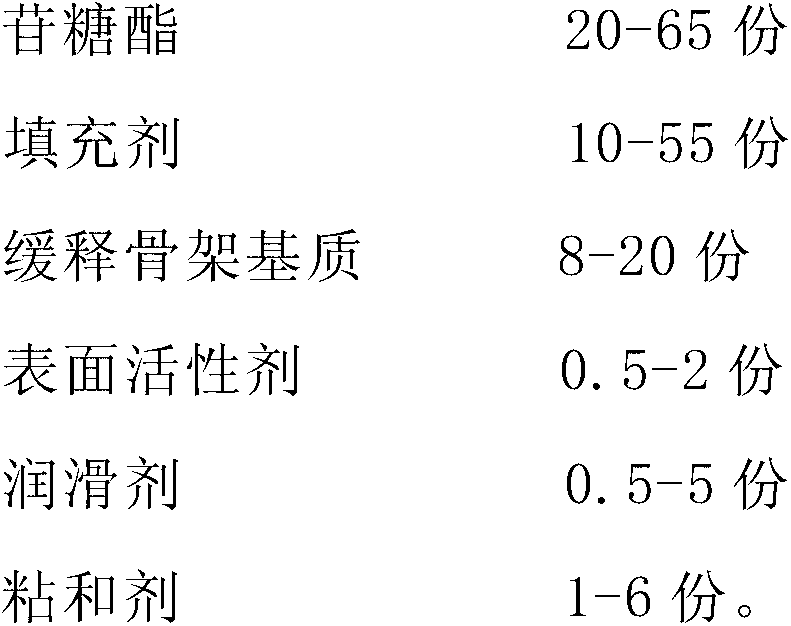
[0020] The preparation of embodiment 1 glycoside sugar ester sustained-release preparation
[0021] Take each component by the following parts by weight:
[0022]
[0023] Mix the glycoside sugar ester, the filler and the sustained-release matrix matrix uniformly; add a binder, make a soft material, dry, and granulate; add a lubricant, mix evenly, and compress into tablets to obtain the product.
Embodiment 2
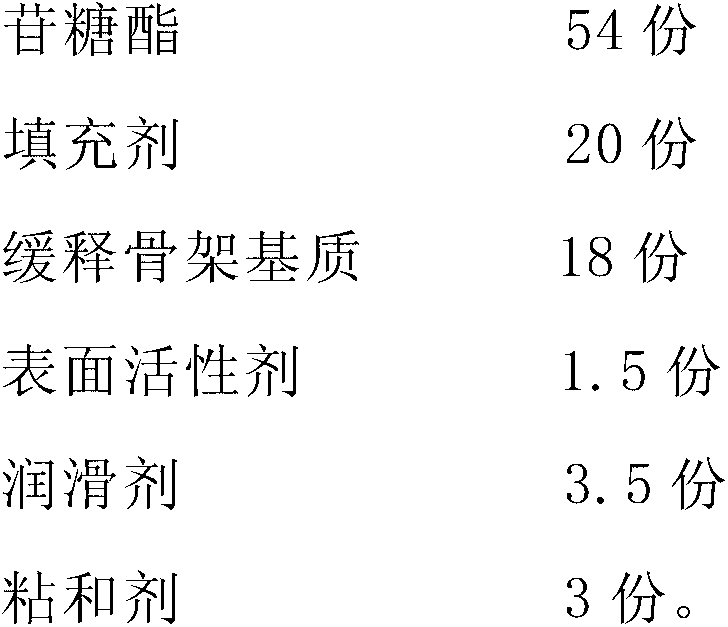
[0024] The preparation of embodiment 2 sugar ester sustained-release preparations
[0025] Take each component by the following parts by weight:
[0026]
[0027] + Mix sugar glycosides, fillers and slow-release matrix matrix methyl cellulose evenly; add binder, make soft material, dry, and granulate; add lubricant, mix evenly, and compress into tablets to obtain the product.
Embodiment 3
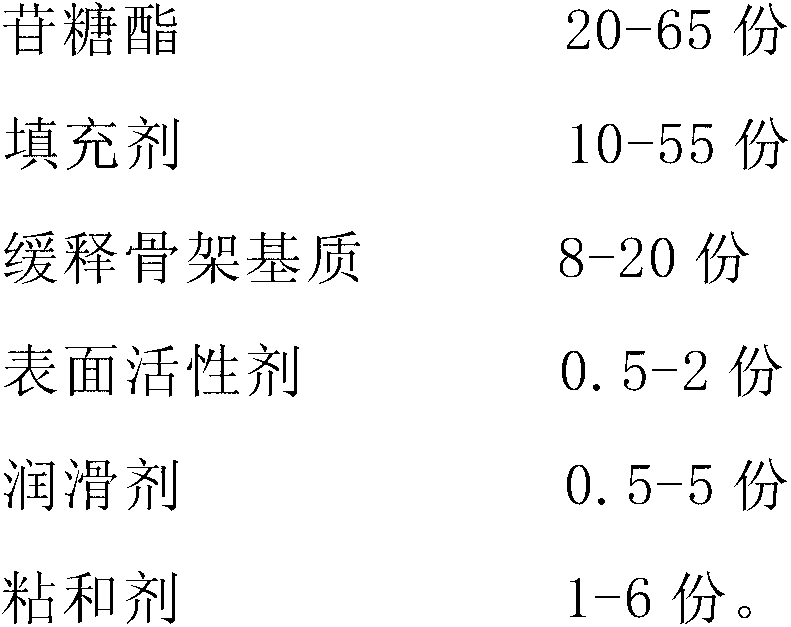
[0028] The preparation of embodiment 3 sugar ester sustained-release preparations
[0029] Take each component by the following parts by weight:
[0030]
[0031] Mix glycoside sugar ester, filler and slow-release matrix matrix hydroxypropyl cellulose uniformly; add binder, make soft material, dry and granulate; add lubricant, mix uniformly, and compress into tablets to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com