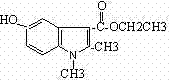
Method for preparing mecarbinate (1,2-dimethyl-5hydroxy-1H-indol-3-ethyl carboxylate)
A technology of mecabene ester and ethyl carboxylate, applied in the field of preparation of mecabene ester (1,2-dimethyl-5 hydroxy-1H-indole-3-carboxylate ethyl ester), can solve Problems such as poor product purity, high cost, and low yield, achieve the effects of low cost, short cycle, and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
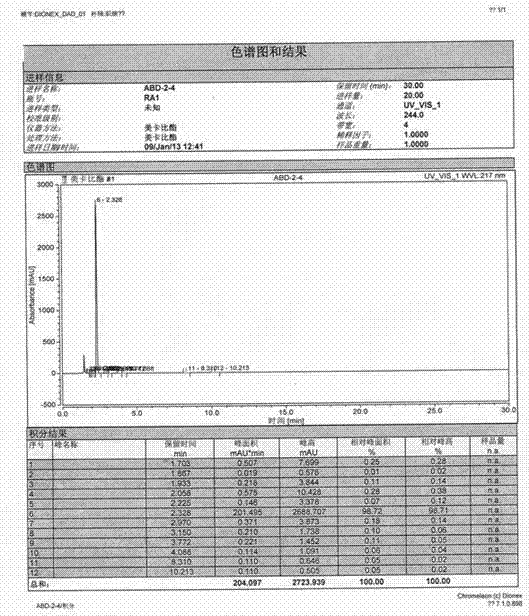
[0025] Add 350g (3.24mol) of p-benzoquinone to 2100ml of dichloromethane, stir at room temperature, add 140g of zinc chloride, heat to 30℃, stir to dissolve completely, control the temperature at 30-35℃ and add 525g (3.67mol) dropwise ) A mixture of ethyl 3-methylamino-2-butenoate in 260ml of dichloromethane, drip in about 1 hour. After the addition is complete, stir and react for 1.5 hours at about 35°C, and then recover about 1400ml of dichloromethane Add 2800ml of 60% ethanol aqueous solution, stir at room temperature for 1 hour, filter, collect the solid, and dry to obtain 648g off-white solid product, yield 85.8%, product mp208-209°C, purity (HPLC) 98.75%. (See figure 1 )
[0026] 1 HNMR (CDCl 3 ,δppm ), 1.36 (t, 3H -CH3), 2.26 (S, 3H -CH3), 3.54 (S.3H-CH3), 4.35 (S.2H -CH2), 6.40-7.10 (m.3H Ar-H ).
[0027] IR(KBr,.cm -1 ) 3560, 3010, 2940, 2860, 1760, 1450, 1250, 1010, 810, 650,
Embodiment 2
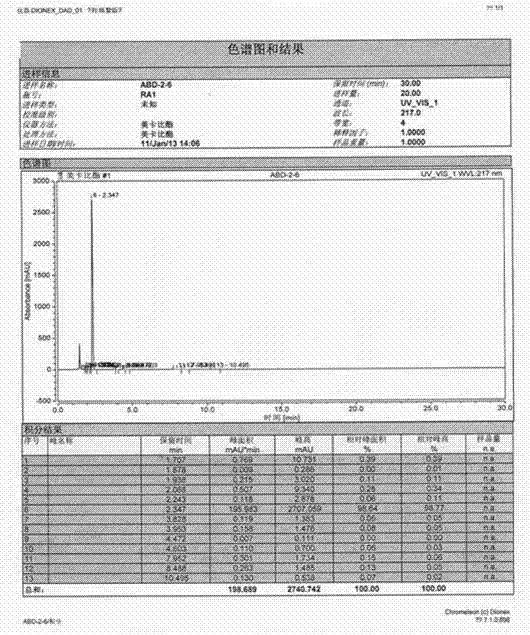
[0029] Add 350g (3.24mol) of p-benzoquinone to 2100ml of 1,2-dichloroethane, stir at room temperature, add 140g of zinc chloride, heat to 30℃, stir to dissolve completely, control the temperature to 35-40℃ and add dropwise 525g (3.67mol) 3-methylamino-2-butenoic acid ethyl ester in 260ml, 1.2-dichloroacetic acid mixed solution, about 1 hour dripping, after the addition, control 35-40 ℃ stirring reaction for 1.5 hours, then control 40 About 1460ml of dichloroethane was recovered under reduced pressure at about ℃, added with 2800ml of 60% ethanol aqueous solution, stirred at room temperature for 1 hour, filtered, collected the solid, and dried to obtain an off-white solid product 642g, mp208-210℃. Yield 85.1%, purity ( HPLC) 98.61% (see figure 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com