Microwave-driven rna polymerization by rna polymerases of caliciviruses
An RNA polymerase and calicivirus technology, which is applied in the field of microwave-driven calicivirus RNA polymerase RNA polymerization, and can solve the problem of not providing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
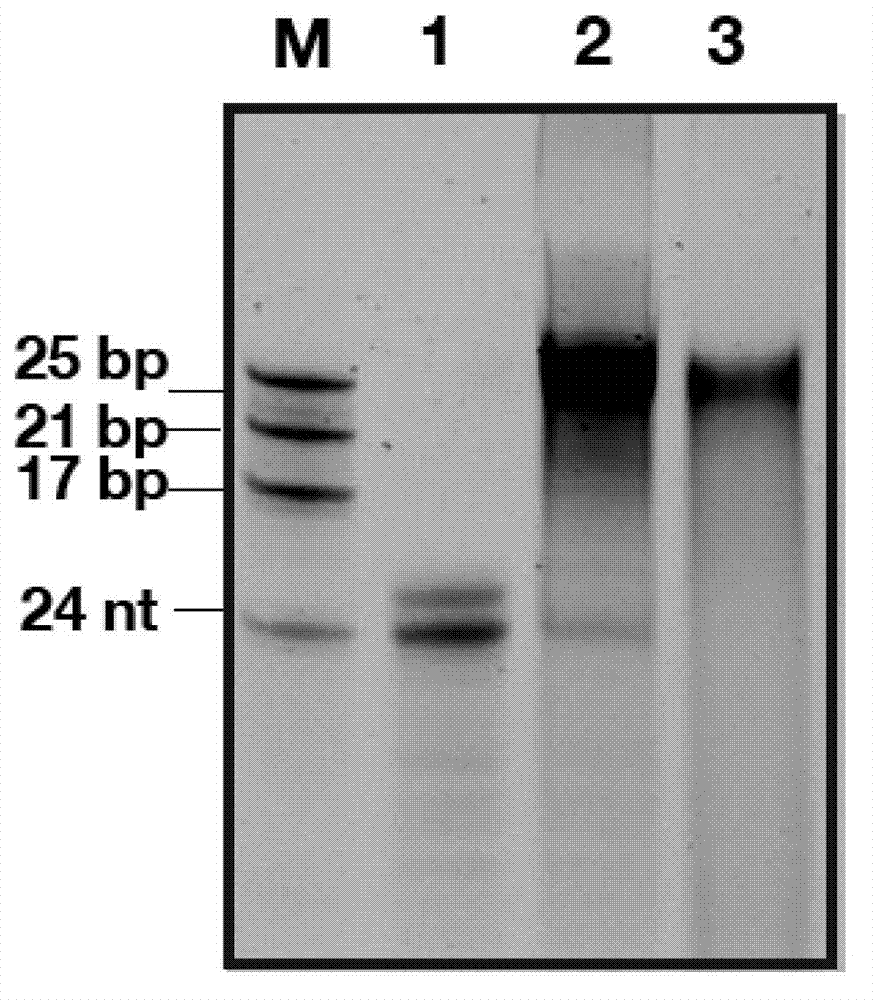
[0074] Example 1: Microwave-driven primer-independent de novo priming of RNA synthesis and double-stranded RNA generation by sapovirus RNA polymerase
[0075] Sapovirus RNA polymerase (SEQ ID NO:11) and RNA template (5'-AUACCUAGAAUCUGACCAACCCCC-3'; SEQ ID NO:15) were irradiated with microwaves at 800W for 60s in a conventional microwave oven. The sapovirus RNA polymerase uses single-stranded RNA as a template to generate double-stranded RNA (Figure 1, lane 2). The resulting product is incubated with S1 nuclease. The product was not digested after incubation with S1 nuclease (Figure 1, lane 3), indicating the double-stranded nature of the product. All reactions were performed in a total volume of 25 μl. The RNA polymerase reaction mix contains 1 μg template, 7.5 μM RNA polymerase, each of 0.4 mM ATP, CTP, UTP, and 2 mM GTP, 5 μl reaction buffer (HEPES 250 mM, MnCl 2 25mM, DTT 5mM, pH7.6), and RNAse-DNAse-free water to make up to a total volume of 25μl. For S1 nuclease dige...
example 2
[0076] Example 2: Microwave Power and Irradiation Time Required for Microwave-Driven RNA Synthesis by Calicivirus RNA Polymerase
[0077] Sapovirus RNA polymerase (SEQ ID NO:11) was incubated with RNA template (5'-AUACCUAGAAUCUGACCAACCCCC-3'; SEQ ID NO:15). The reaction was irradiated in a conventional microwave oven at 80W, 160W, 240W, 320W, 400W, 480W, 560W, 640W, 720W and 800W for 60 s in a total volume of 25 μl. In another experiment, the reactant was irradiated for 60s, 30s, 15s, or 5s in the same microwave oven at 80W (as shown in Figure 2B, lanes 1-4), or irradiated at 800W for 60s, 30s, 15s, or 5s ( Figure 2B, lanes 5-8). Products of the expected size were produced in all reactions (Fig. 2A, 2B).
[0078] The reaction mix contains 1 μg template, 7.5 μM RNA polymerase, each of 0.4 mM ATP, CTP, UTP, and 2 mM GTP, 5 μl reaction buffer (HEPES 250 mM, MnCl 2 25mM, DTT 5mM, pH 7.6), and RNAse-DNAse-free water to make up to a total volume of 25μl. The product was separat...
example 3
[0079] Example 3: Calicivirus RNA polymerase de novo primes RNA synthesis in a primer-independent manner using DNA as a template under microwave irradiation, and incorporates 2'-fluoro-GMP into double-stranded DNA / RNA products
[0080] Sapovirus RNA polymerase (SEQ ID NO:11) was incubated with ssDNA template (5'-ATACCTAGAATCTGACCAACCCCC-3'; SEQ ID NO:16) or with the same sequence but with (rC) at the 3' end 5 DNA template for the motif (5'-ATACCTAGAATCTGACCAArCrCrCrCrC-3'; SEQ ID NO: 17). As a control, sapovirus RNA polymerase was incubated with single-stranded RNA (5'-AUACCUAGAAUCUGACCAACCCCC-3'; SEQ ID NO: 15) having the same sequence as the aforementioned single-stranded DNA. All reactions were irradiated in a conventional microwave oven at 800W for 60 s in a total volume of 25 μl. The reaction mix contained 1 μg template, 7.5 μM RNA polymerase, 0.4 mM each of ATP, CTP, UTP, and GTP (using ssRNA template as a control reaction; Figure 3, lane 3) or 2'-fluoro-GTP (reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com