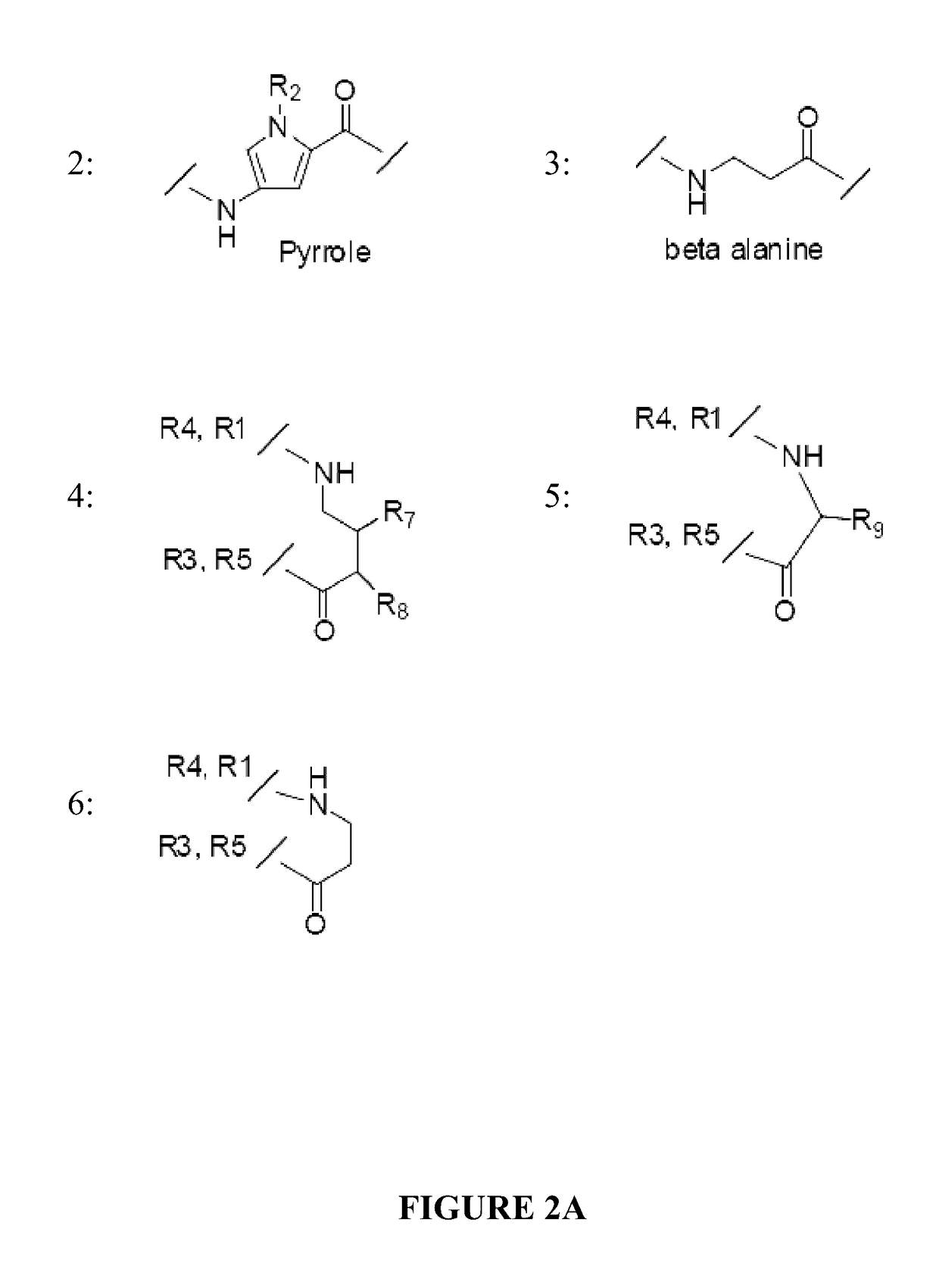
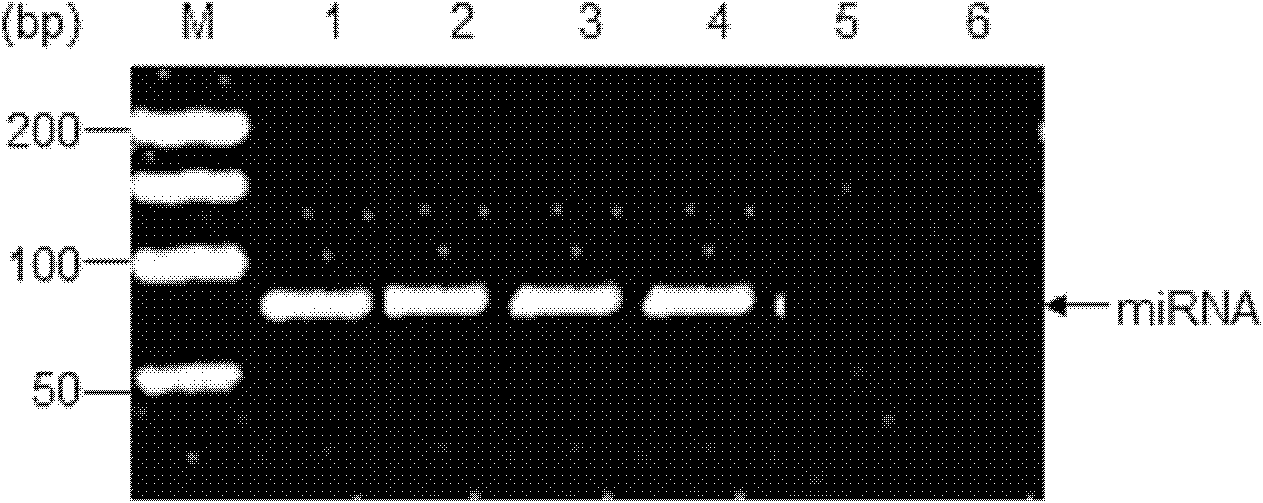Patents
Literature
40 results about "RNA polymerase II" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
RNA polymerase II (RNAP II and Pol II) is a multiprotein complex. It is one of the three RNAP enzymes found in the nucleus of eukaryotic cells. It catalyzes the transcription of DNA to synthesize precursors of mRNA and most snRNA and microRNA. A 550 kDa complex of 12 subunits, RNAP II is the most studied type of RNA polymerase. A wide range of transcription factors are required for it to bind to upstream gene promoters and begin transcription.
Methods and compositions for the production of guide RNA
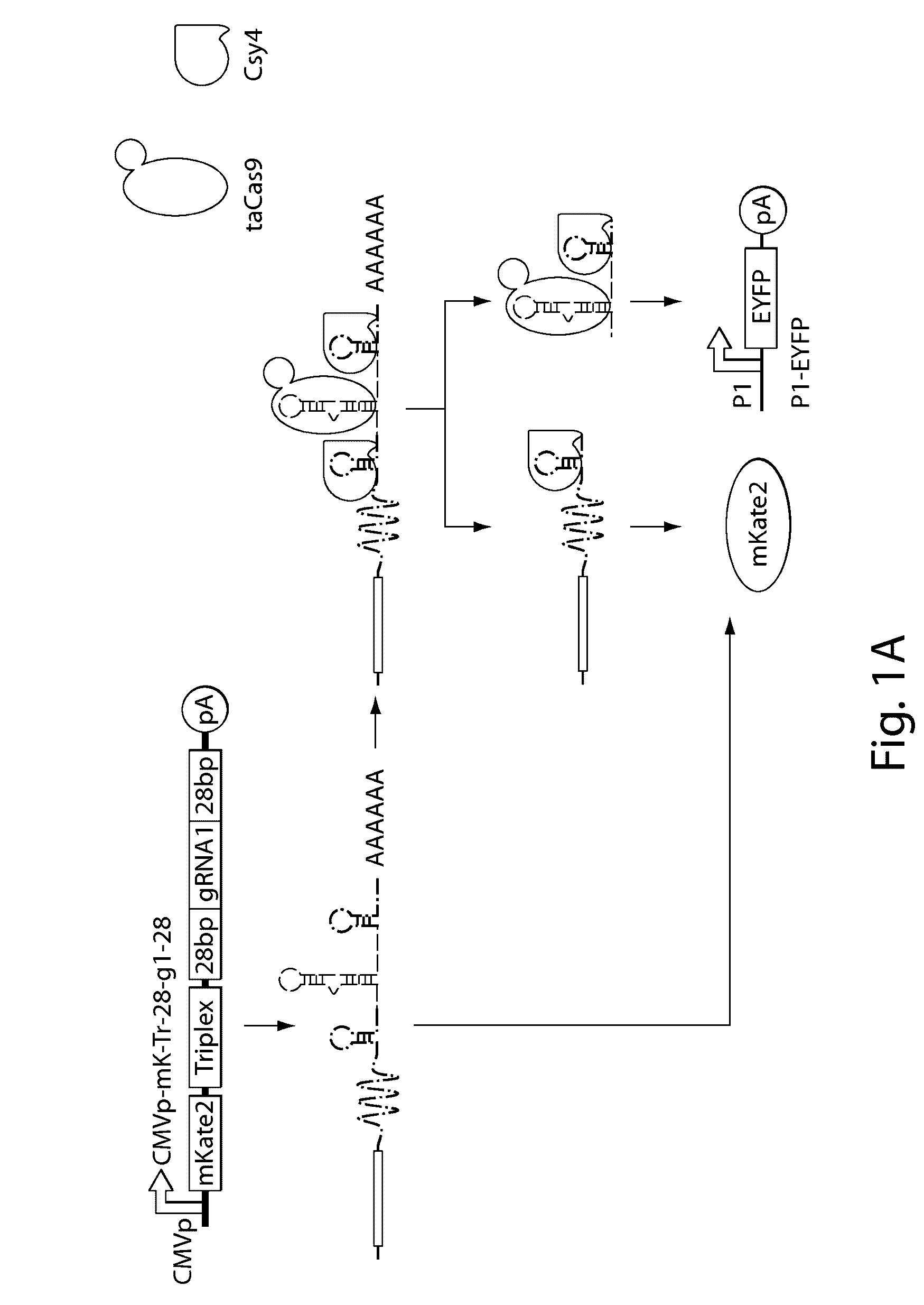
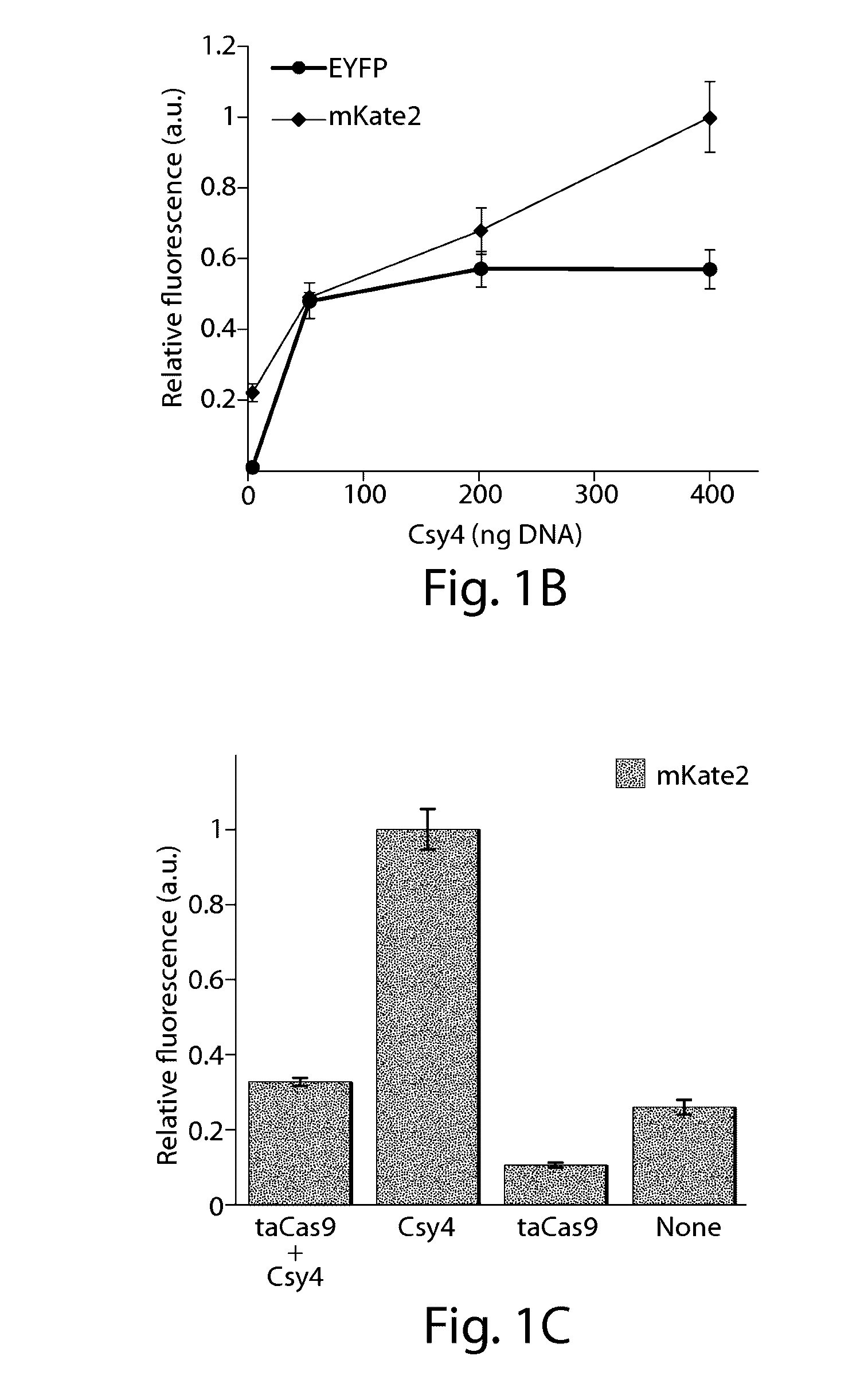
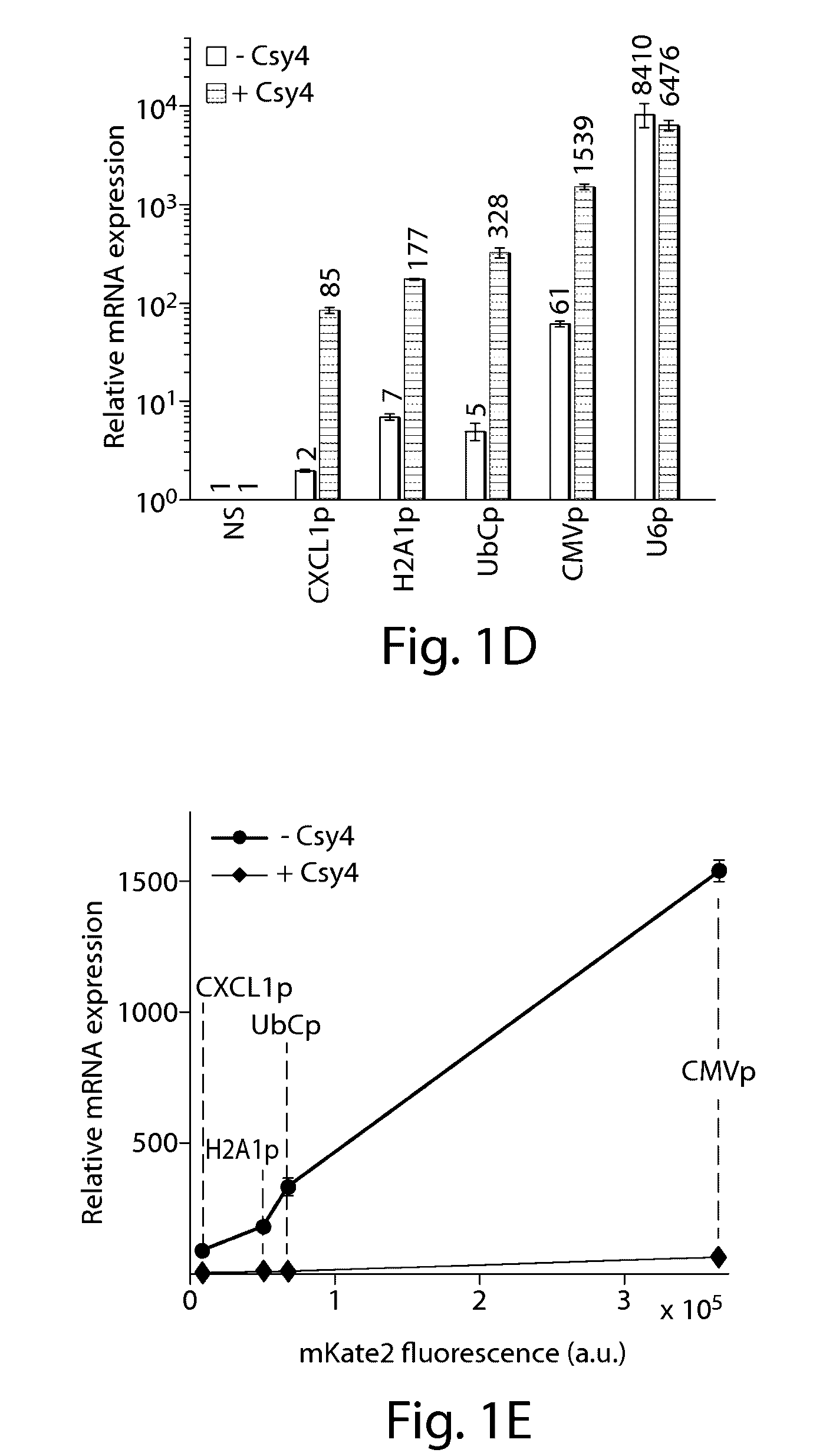
Various aspects and embodiments of the present disclosure relate to methods and compositions that combine multiple mammalian RNA regulatory strategies, including RNA triple helix structures, introns, microRNAs, and ribozymes with Cas-based CRISPR transcription factors and ribonuclease-based RNA processing in human cells. The methods and compositions of the present disclosure, in some embodiments, enable multiplexed production of proteins and multiple guide RNAs from a single compact RNA-polymerase-II-expressed transcript for efficient modulation of synthetic constructs and endogenous human promoters.
Owner:MASSACHUSETTS INST OF TECH
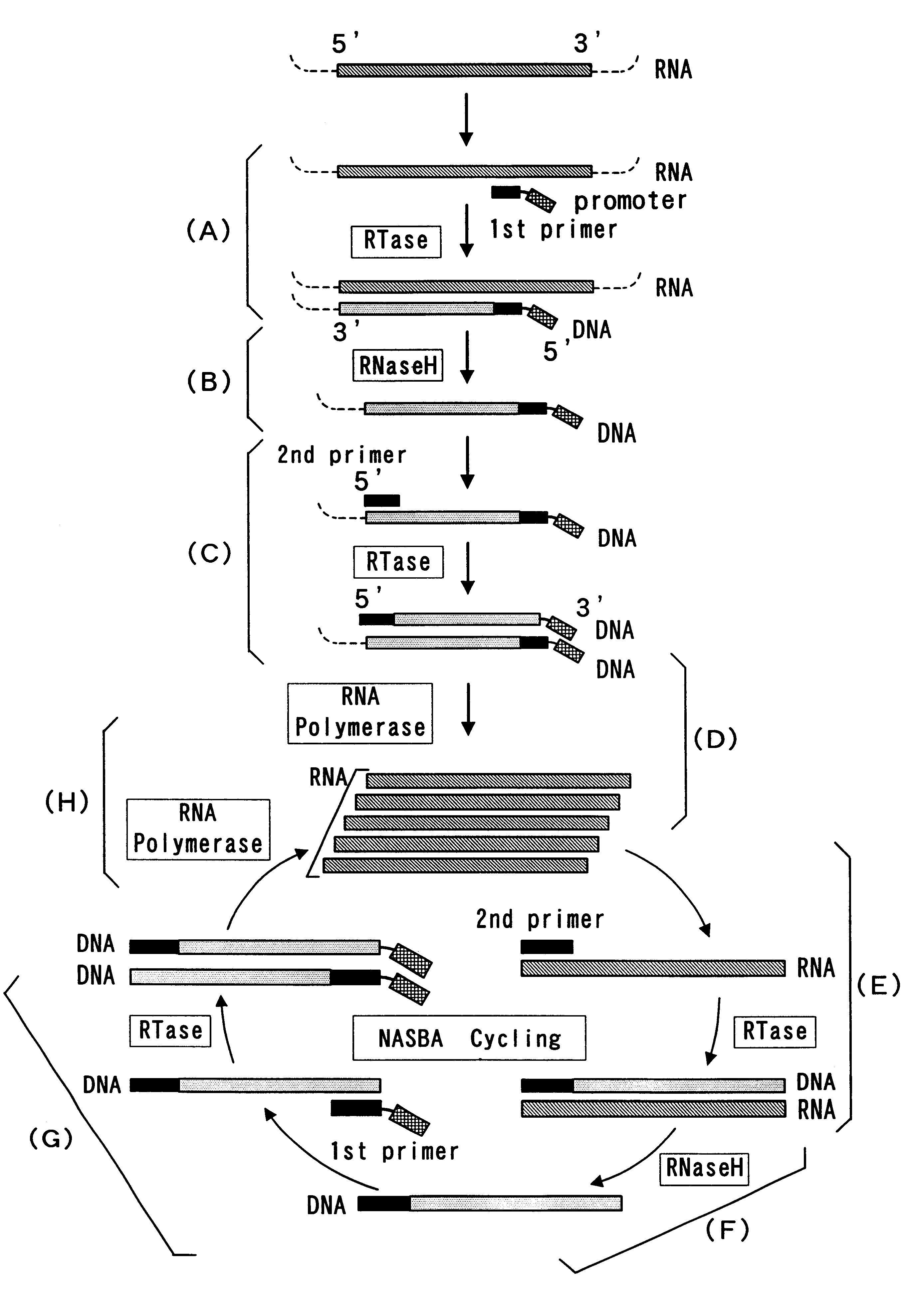
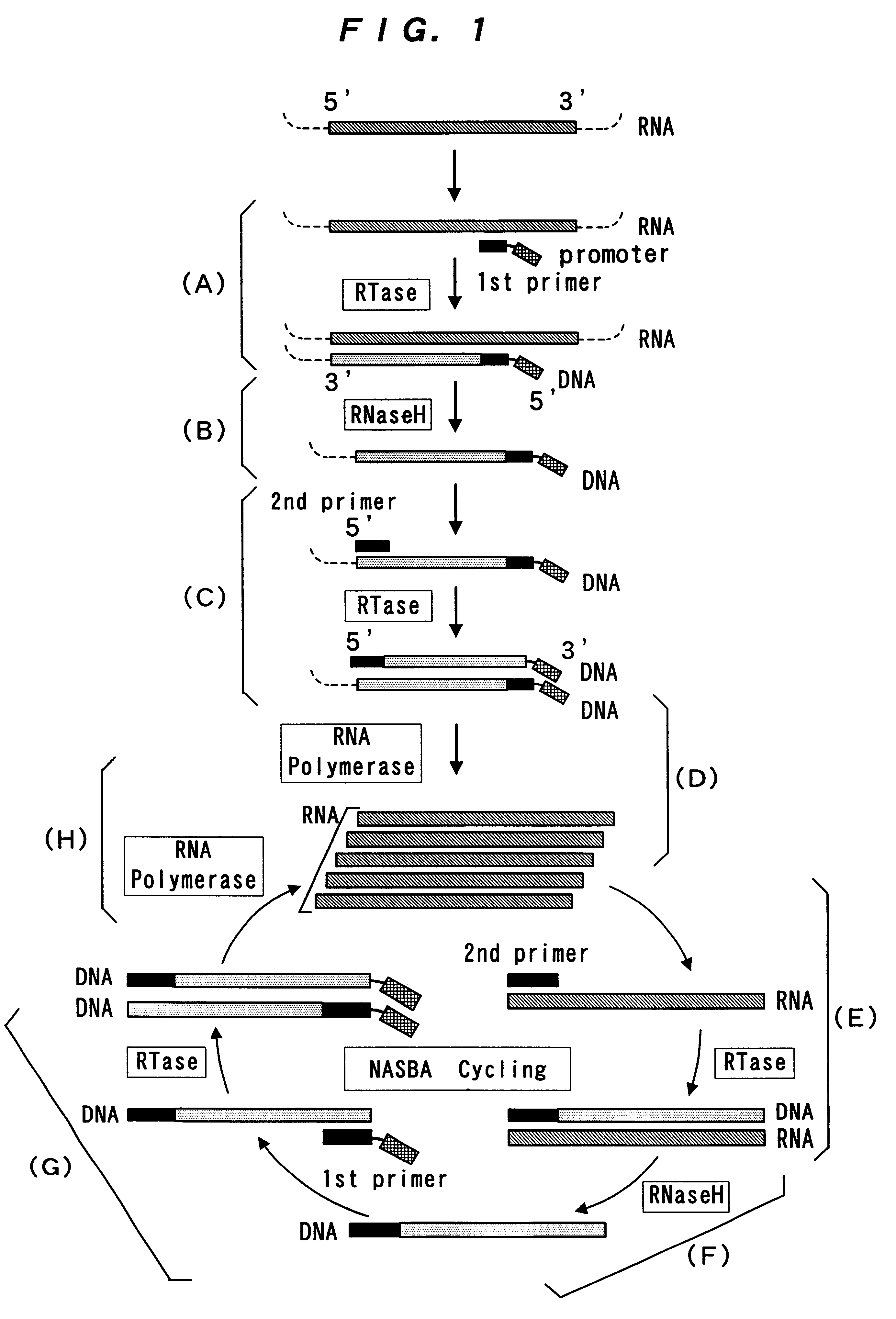
Reagent for nucleic acid amplification and process for nucleic acid amplification
InactiveUS6261773B1Microbiological testing/measurementRecombinant DNA-technologyForward primerPolymerase L
The present invention provide a process for sequence-specific nucleic acid amplification capable of improving the detection sensitivity and increasing the signal. In particular, the present invention provides a reagent for nucleic acid amplification containing at least one member selected from the group consisting of EDTA, NTA, UDA, CyDTA, DTPA, GEDTA, TTHA and their salts, specifically a reagent for nucleic acid amplification comprising, in addition to the at least one compound, a forward primer having a DNA sequence homologous to a sequence of a target RNA; a reverse primer having a DNA sequence complementary to a sequence of the target RNA and having a promoter for RNA polymerase attached to its 5' end; ribonucleotides; deoxyribonucleotides; a reverse transcriptase or RNA-directed DNA polymerase; a RNase H; a DNA polymerase or a reverse transcriptase having DNA-directed DNA polymerase activity; and a RNA polymerase. The present invention also provides a process for nucleic acid amplification characterized by carrying out a nucleic acid amplification reaction in the presence of at least one member selected from the group consisting of EDTA, NTA, UDA, CyDTA, DTPA, GEDTA, TTHA and their salts.
Owner:TOYOBO CO LTD
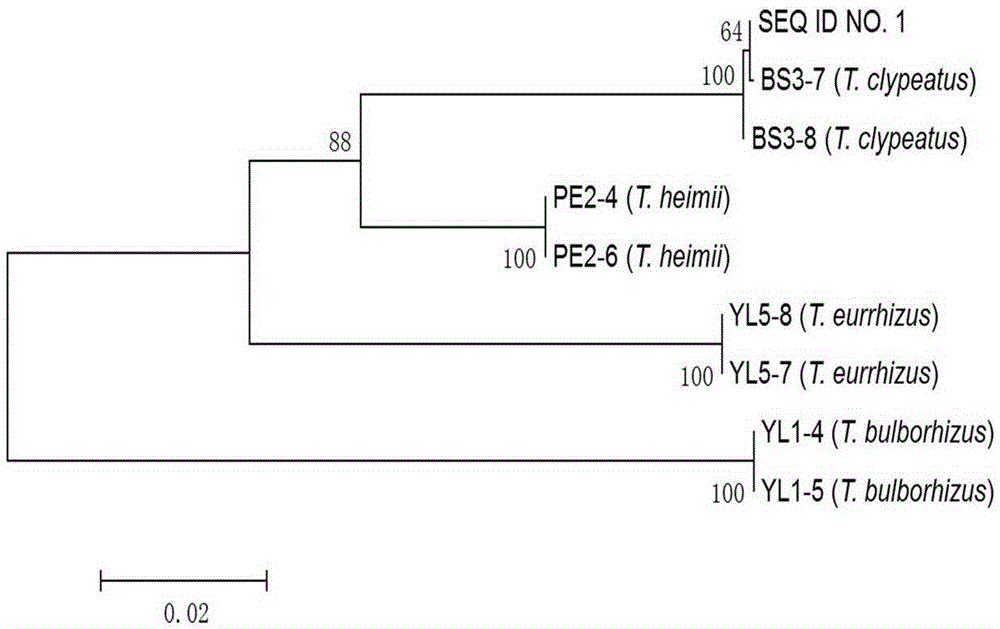
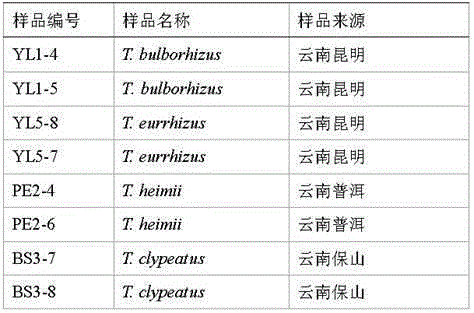
DNA (deoxyribonucleic acid) barcode reference gene of termitomyces clypeatus and application of DNA barcode reference gene of termitomyces clypeatus
InactiveCN105238794AEasy to expandEasy to compareMicrobiological testing/measurementGenetic engineeringMolecular identificationReference genes
The invention belongs to the field of fungus species identification and relates to a reference gene for molecular identification of Termitomyces clypeatus and a molecular identification method. Sequences of adopted primers are RPB2B-f: 5'-CCGCAAAGGCTGGTGTATC-3' and RPB2B-r: 5'-TTCGAATGAGTTCAAGTGT-3', and the primers can be used for amplification of large-subunit gene (RPB2) of RNA (ribonucleic acid) polymerase II of the termitomyces clypeatus to obtain a reference detection gene. The termitomyces clypeatus can be quickly and accurately identified according to RPB2 gene DNA (deoxyribonucleic acid) sequence differences. The RPB2 gene overcomes the defect of difficulty in identification of traditional termitomyces clypeatus forms and has the advantages of universality and easiness in amplification and comparison, reliability and accuracy in identification are greatly improved, and a powerful research implement is provided for excavation, protection and utilization of genetic resources of the termitomyces clypeatus.
Owner:YUNNAN UNIV
Application of protein molecular marker in fish germ cell development research
ActiveCN111487399AConducive to filling the technical gaps in the developmental stateBiological testingFluorescence/phosphorescenceImmunofluorescenceProtein molecules
The invention provides an application of a protein molecular marker in fish germ cell development research. The protein molecule is Anti-RNA polymerase II CTD repeat YSPTSPS (Phospho S5), and the application comprises the step of carrying out immunofluorescence localization analysis on fishes in different development states by adopting the protein molecule as a marker molecule. The immune probe isapplied to fish germ cell development research for the first time, cell facies characteristics of different fish germ cell developments are comprehensively analyzed, an important molecular marker isprovided for judging the development state of the fish germ cells, and a new development function detection strategy is provided for fish genetic breeding.
Owner:HUNAN NORMAL UNIVERSITY
Untranslated region-specific artificial microRNAs that effectively inhibit the replication of different strains of porcine reproductive and respiratory syndrome virus
The invention designs an untranslated region specific artificial micro RNA (miRNA) capable of effectively inhibiting replication of porcine reproductive and respiratory syndrome (PRRS) virus strains, which belongs to the field of researches on gene engineering and biological products. The sequence of the miRNA is represented by one of SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 and SEQ ID No.4. The invention also discloses the construction of an artificial miRNA expression vector based on RNA polymerase II, design of miRRNAi, synthesis and cloning of double-stranded oligo, detection of expression of miRNA and inhibition effect on replication of different PRRSV strains. Compared with siRNAs for genes of other PRRSVs, the artificial miRNA screened by the invention, according to the conservative sequence of the untranslated region, can inhibit the replication of homologous virus strains and heterologous virus strains and can be used in both the development of anti-PRRSV infection preparations and breeding transgenic pig disease resistance lines.
Owner:YANGZHOU UNIV
Reproduction of ribonucleic acids
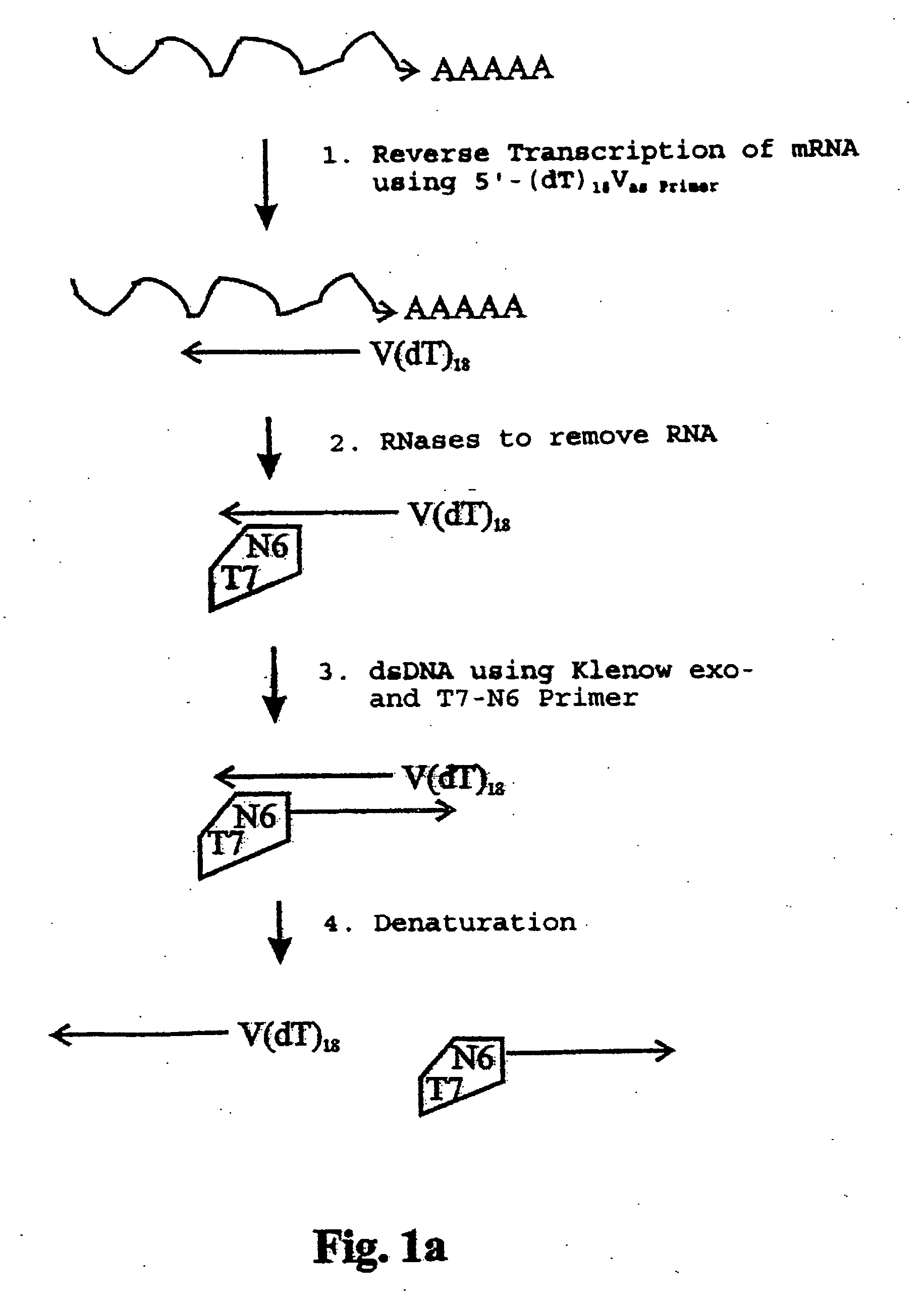
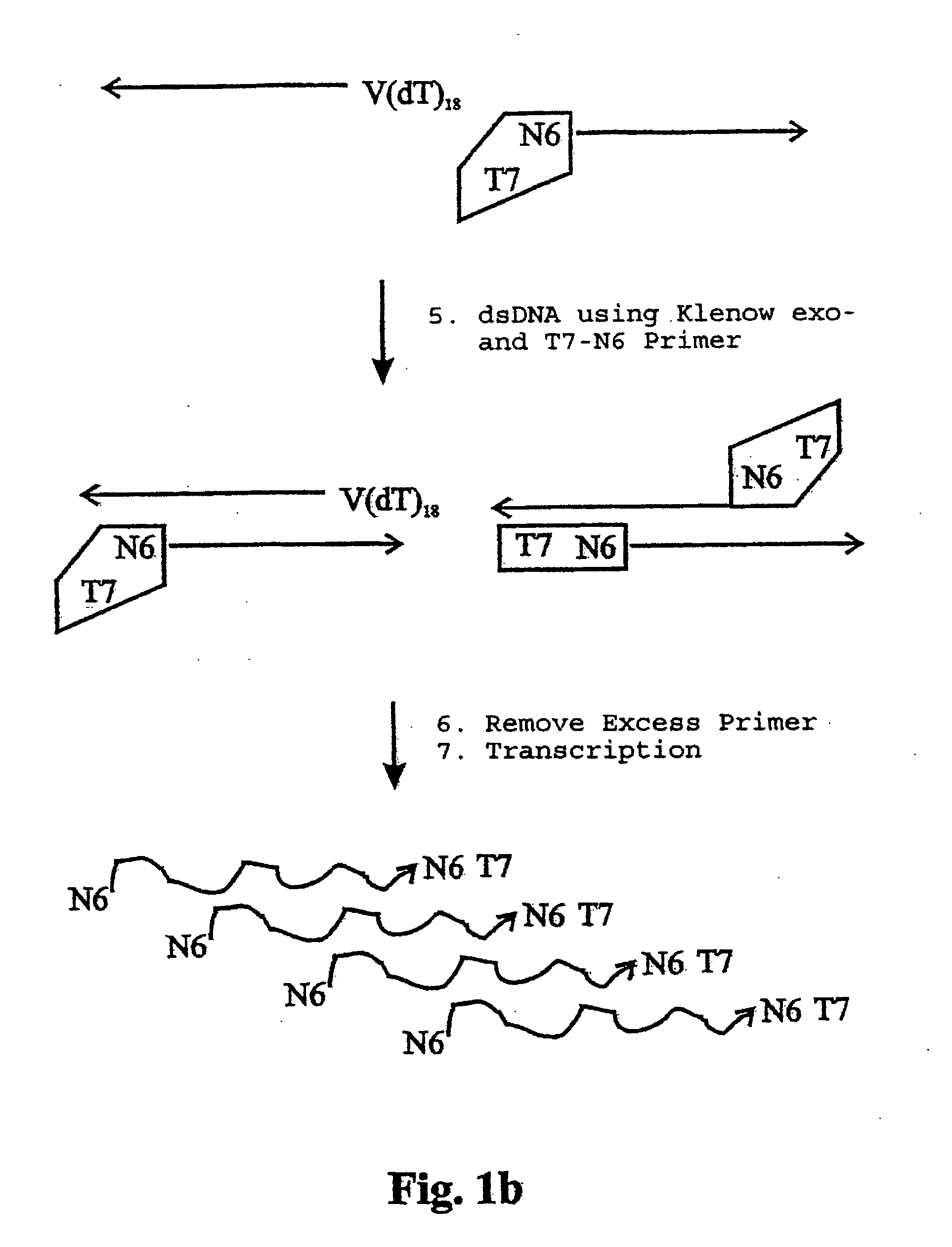
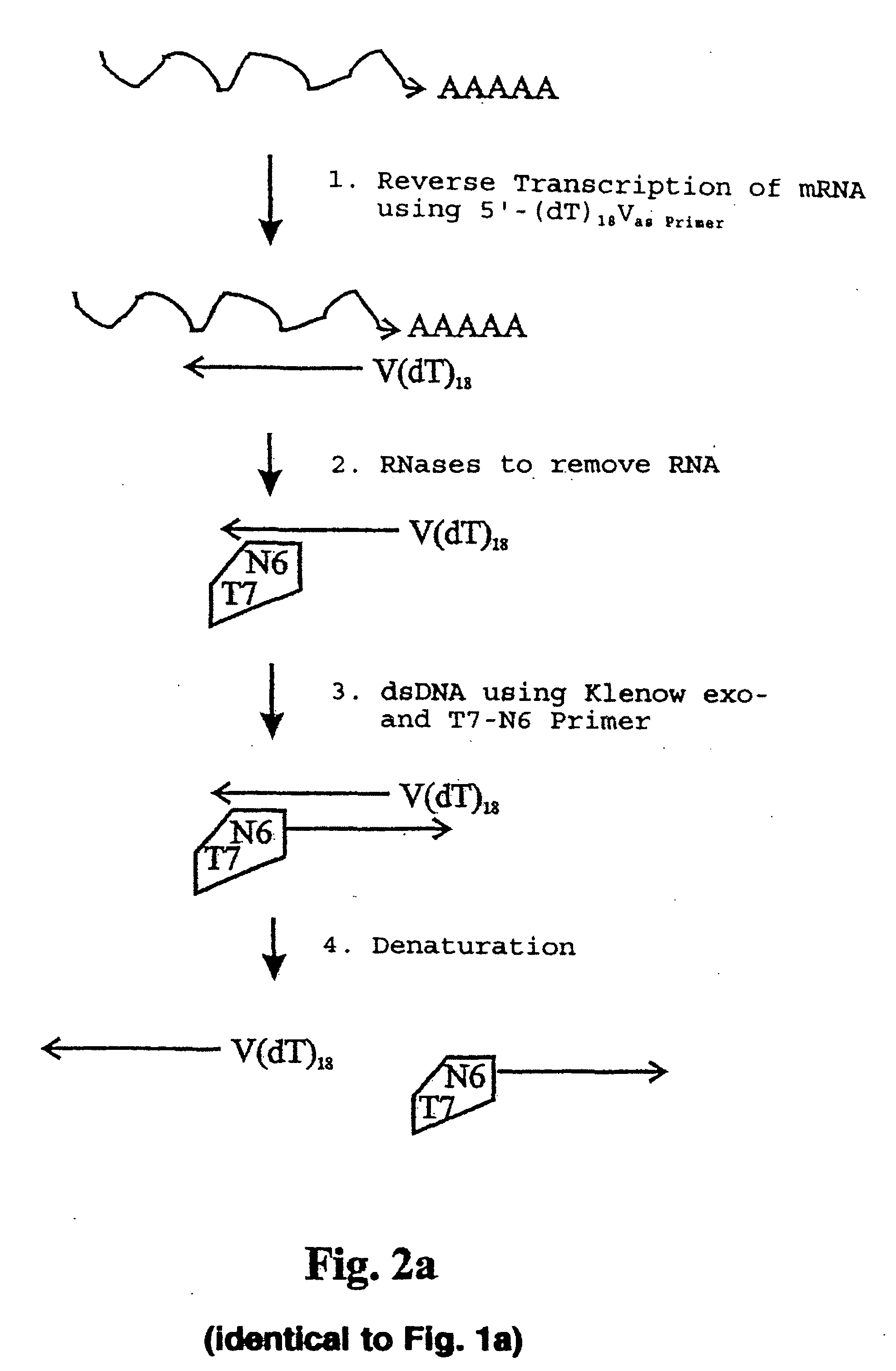
InactiveUS20050009027A1Microbiological testing/measurementBiological testingSingle-Stranded RNAPolymerase L
The present application relates to processes resulting in the amplification of ribonucleic acids. The processes comprise the following steps: (a) using a single stranded primer, an RNA-dependent DNA polymerase and deoxyribonucleotide monomers to synthesize a single stranded DNA via reverse transcription of RNA; (b) removing of the RNA; (c) using a single stranded primer comprising a promoter sequence, a DNA polymerase and deoxyribonucleotide monomers to synthesize a double stranded DNA; (d) separating the double stranded DNA into single stranded DNAs; (e) using a single stranded primer comprising a promoter sequence, a DNA polymerase and deoxyribonucleotide monomers to synthesize double stranded DNA on the basis of the single stranded DNA obtained in (d); (f) using an RNA polymerase and ribonucleotide monomers to synthesize multiple single stranded RNAs.
Owner:AMPTEC
Method for constructing molecularly marked CSFV (classical swine fever virus) attenuated vaccine
ActiveCN105886531AEfficient and Stable RescueGood genetic stabilitySsRNA viruses positive-senseAntiviralsAnti virusBovine Viral Diarrhea Viruses
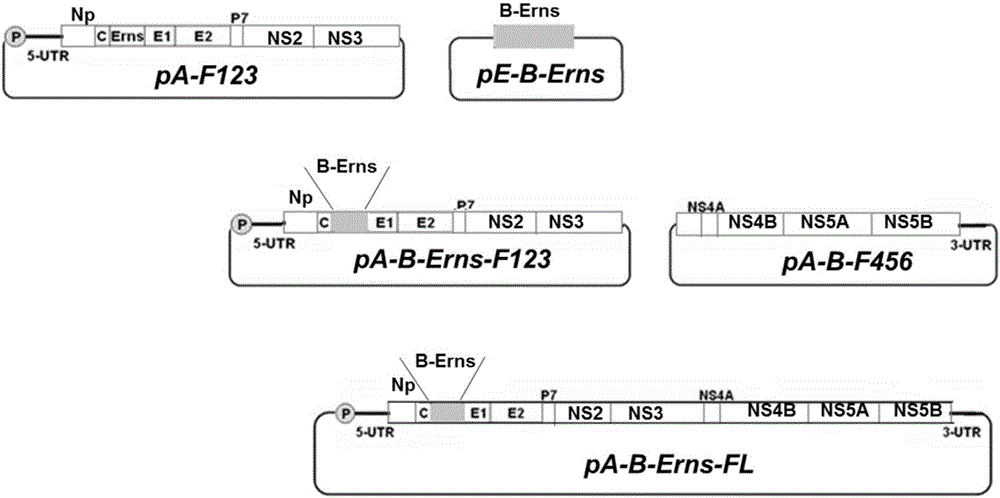
The invention relates to the field of biotechnology and aims to provide a method for constructing a molecularly marked CSFV (classical swine fever virus) attenuated vaccine. The method comprises following steps: a recombinant plasmid pA-F123 containing genome 5' half-length of a CSFV vaccine strain C and a recombinant plasmid pE-B-Erns containing Erns genes of a BVDV (bovine viral diarrhea virus) strain VEDEVAC are taken as templates, 20bp of homologous fragments are designed at two ends, and by means of a one-step orient cloning kit, a recombinant plasmid pA-B-Erns-F123 is obtained; an F456 fragment containing genome 3' half-length of the CSFV vaccine strain C is cut off from a recombinant plasmid pB-F456 through BamH I and Sal I and cloned into pA-B-Erns-F123 under the action of the same enzyme, and a recombinant plasmid pA-B-Erns-FL is obtained. By virtue of establishment of a reverse genetic manipulation technology, a new way is developed for research of CSFV gene functions and novel vaccines. An exogenous tag is inserted into a viral genome with the reverse genetic manipulation technology for research of virus replication, virus encoded protein functions and anti-virus drug screening, and an RNA (ribonucleic acid) polymerase II system can efficiently and stably save CSFV infectious cDNA (complementary deoxyribonucleic acid ) cloning.
Owner:ZHEJIANG UNIV
Recombinant hog cholera virus for expressing firefly luciferase gene and application of recombinant hog cholera virus
InactiveCN104017779AMicrobiological testing/measurementViruses/bacteriophagesHigh-Throughput Screening MethodsMicrobiological culture
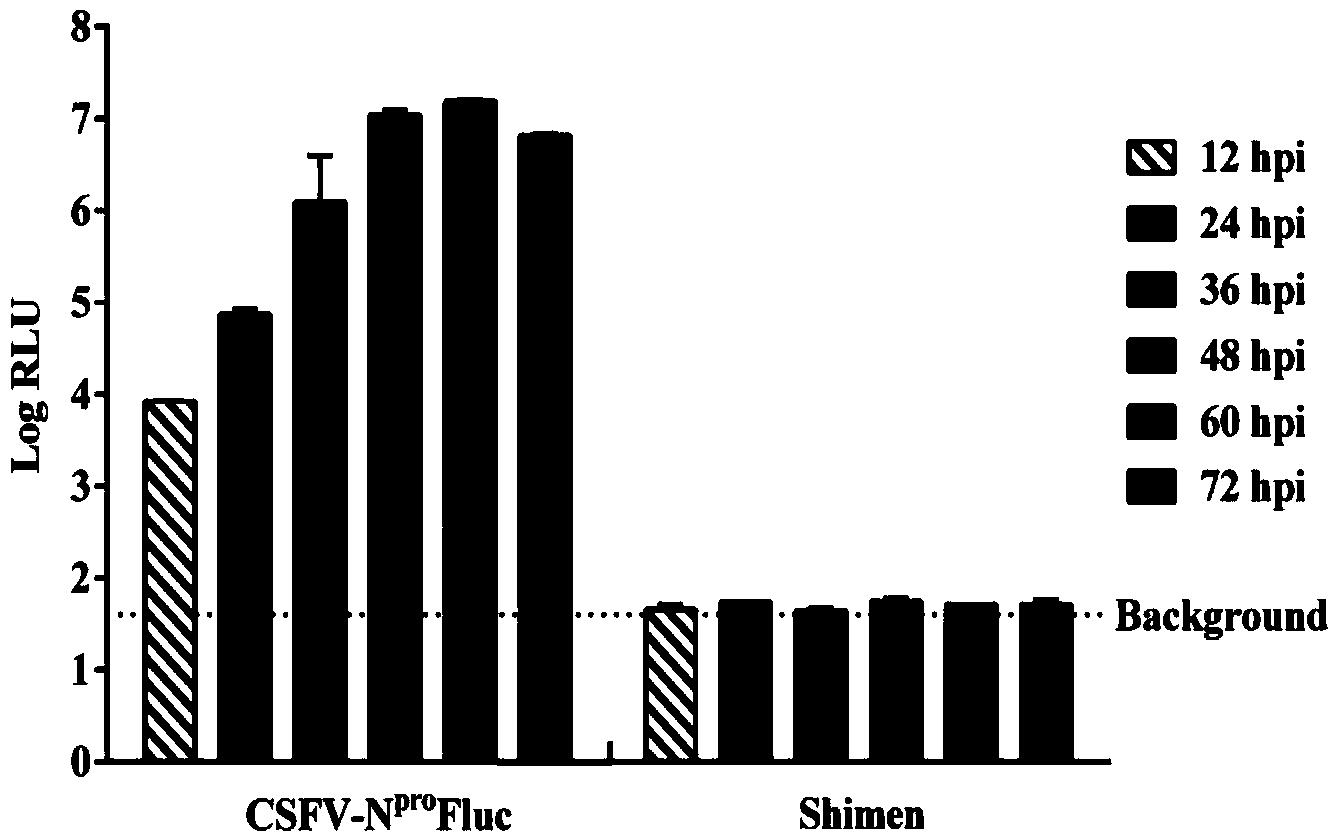
The invention discloses a recombinant hog cholera virus for expressing firefly luciferase gene and application of the recombinant hog cholera virus, and belongs to the field of rescuing and applying recombinant hog cholera virus for expressing firefly luciferase gene. According to the recombinant hot cholera virus, a firefly luciferase gene is cloned into a Npro protein coding region of a hog cholera virus Shimen strain full infectious cloned pBRCISM by an overlapping polymerase chain reaction method, the recombinant virus CSFV-NproFluc capable of stably expressing firefly luciferase gene is rescued by utilizing an RNA polymerase II reverse genetic operation technology, wherein the preservation number of the recombinant hog cholera virus is CGMCC (China General Microbiological Culture Collection Center) No.9058. The firefly luciferase gene carried by the recombinant hog cholera virus can be stably inherited in the continuous passage process, the hog cholera virus infection does not change. The recombinant hog cholera virus has a wide application prospect in antibody titre determination and high-throughput screening hog cholera virus specificity inhibitors and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
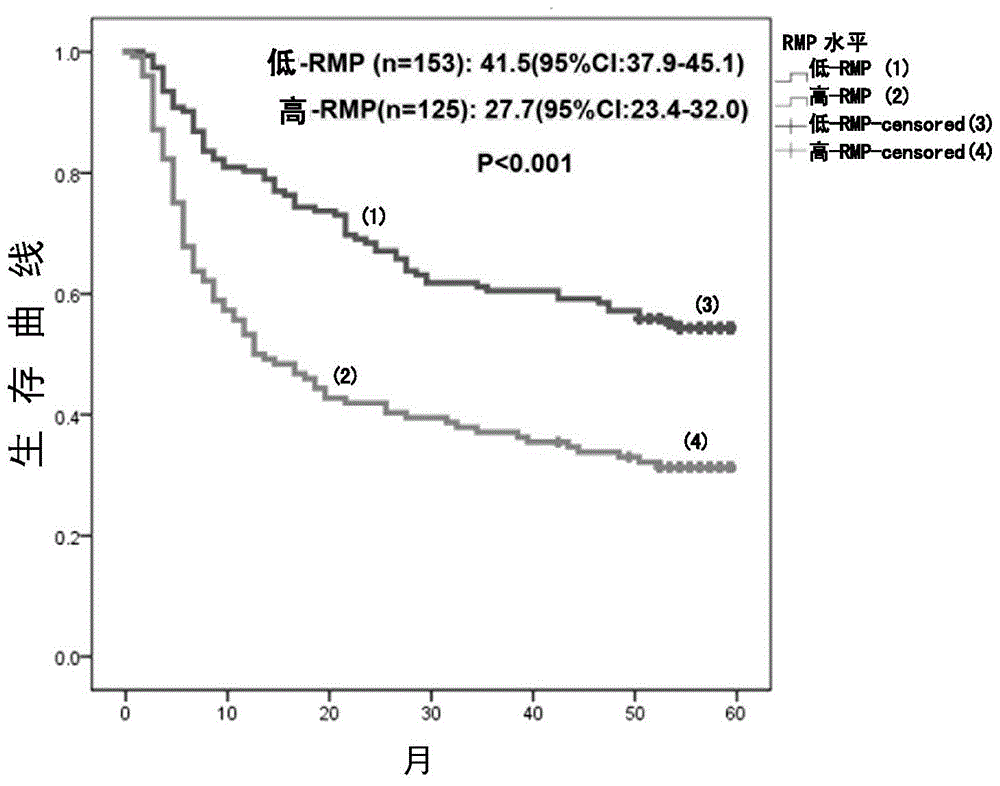
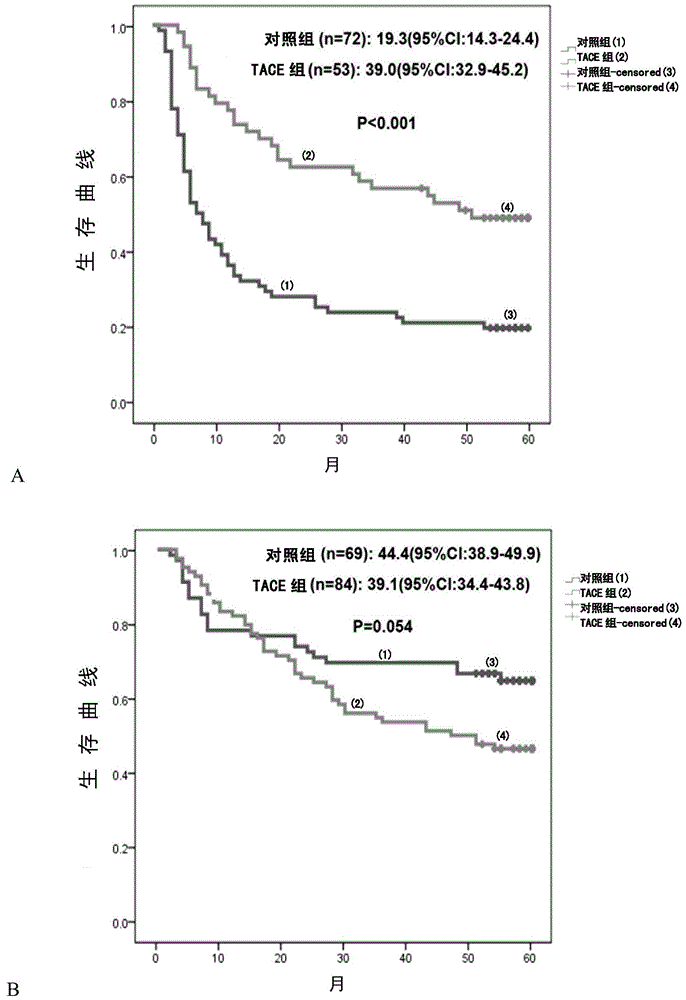
Application of rna polymerase ii fifth subunit regulatory protein in preparation of reagents for hepatocellular carcinoma prognosis or auxiliary tace prognosis
The invention relates to an application of an RNA (Ribose Nucleic Acid) polymerase II fifth subunit regulatory protein (RMP) in preparation of a reagent for prognosis of hepatocellular carcinoma or auxiliary TACE (Transcatheter Arterial Chemoembolization). The invention firstly reveals that high expression of the RMP in a hepatic cell cancer indicates unsatisfactory prognosis of resection of hepatocellular carcinoma after operation; meanwhile, the high expression of the RMP further indicates that the auxiliary TACE after operation is effective. Thus, the invention establishes an RMP-based prognosis method of the hepatic cell cancer after operation.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
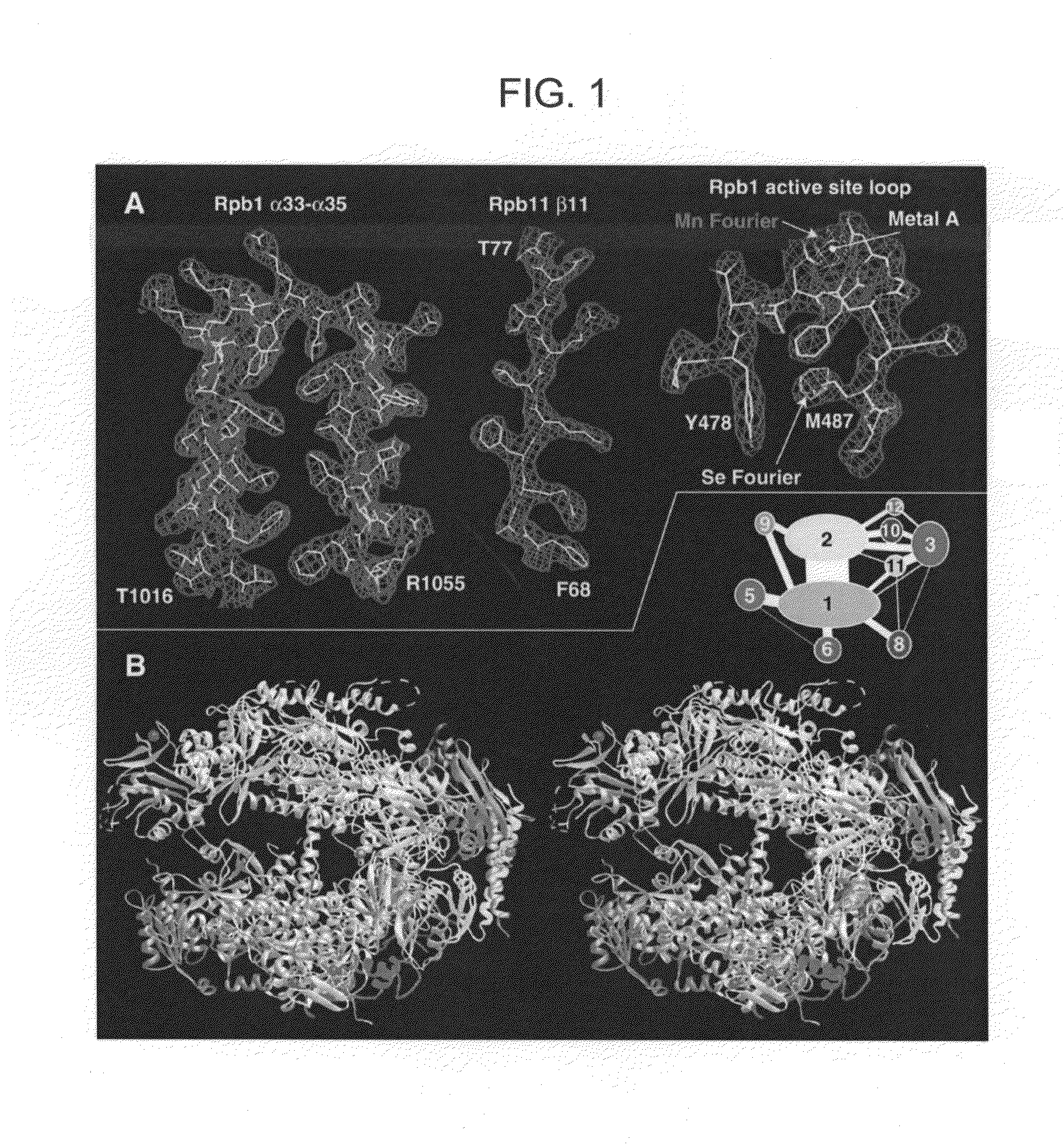
Computer comprising three-dimensional coordinates of a yeast RNA polymerase II
Crystals and structures are provided for an eukaryotic RNA polymerase, and an elongation complex containing a eukaryotic RNA polymerase. The structures and structural coordinates are useful in structural homology deduction, in developing and screening agents that affect the activity of eukaryotic RNA polymerase, and in designing modified forms of eukaryotic RNA polymerase. The structure information may be provided in a computer readable form, e.g. as a database of atomic coordinates, or as a three-dimensional model. The structures are useful, for example, in modeling interactions of the enzyme with DNA, RNA, transcription factors, nucleotides, etc. The structures are also used to identify molecules that bind to or otherwise interact with structural elements in the polymerase.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
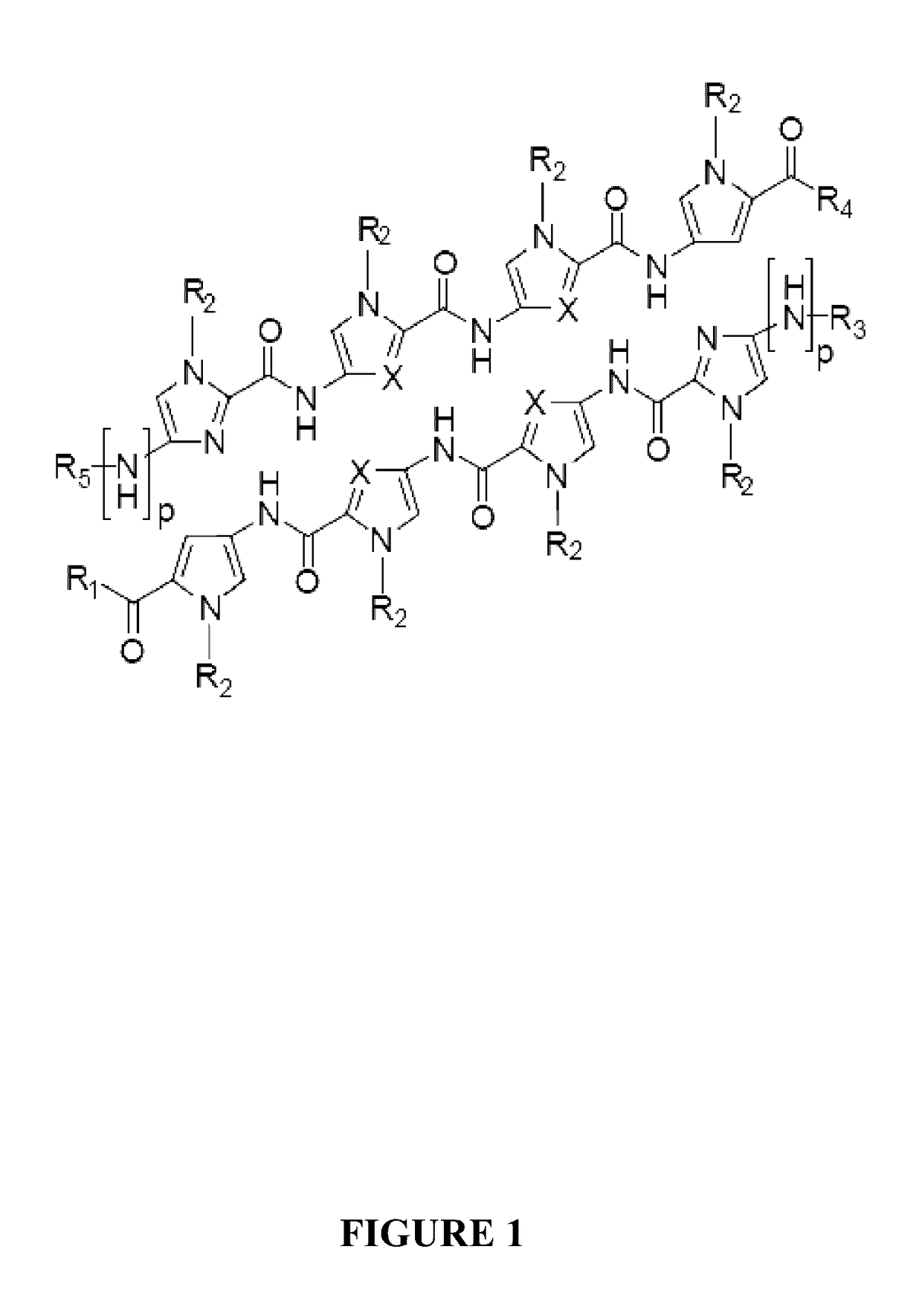
Inhibitors for steroid response elements and RNA polymerase II and related methods
The present invention relates to polyamides capable of inhibiting ARE-, GRE- and ERE-mediated gene regulation in cells. The present invention also relates to polyamides capable of modulating the activity of RNA polymerase II and p53. The invention also relates to methods to treat diseases related to ARE-, GRE- and ERE-mediated gene regulation and to RNA polymerase II and p53 activity.
Owner:CALIFORNIA INST OF TECH
Gene expression down-regulated vector of cell specificity ndrg2
InactiveCN101565717ADown-regulation effect is obviousEasy to observeVector-based foreign material introductionPreproinsulinRNA polymerase II
The invention discloses a gene expression down-regulated vector of cell specificity ndrg2, which comprises an RNAi transgenic vector. A preproinsulin promoter is connected to the RNAi transgenic vector, a miR accessed locus is inserted to the down stream of the preproinsulin promoter, and the miR accessed locus is connected with a miRNA sequence aiming at down-regulated ndrg2 genes. The constructed RNAi transgenic vector adopts the preproinsulin promoter relied by RNA polymerase II to promote the downstream miRNA sequence by specific insulin beta cells of the preproinsulin promoter, and the specific insulin beta cells promote RNA interference aiming at ndrg2 genes to down-regulate the expression thereof; and the vector is used for constructing an animal model and researching the functions of the ndrg2 genes of the insulin beta cells.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
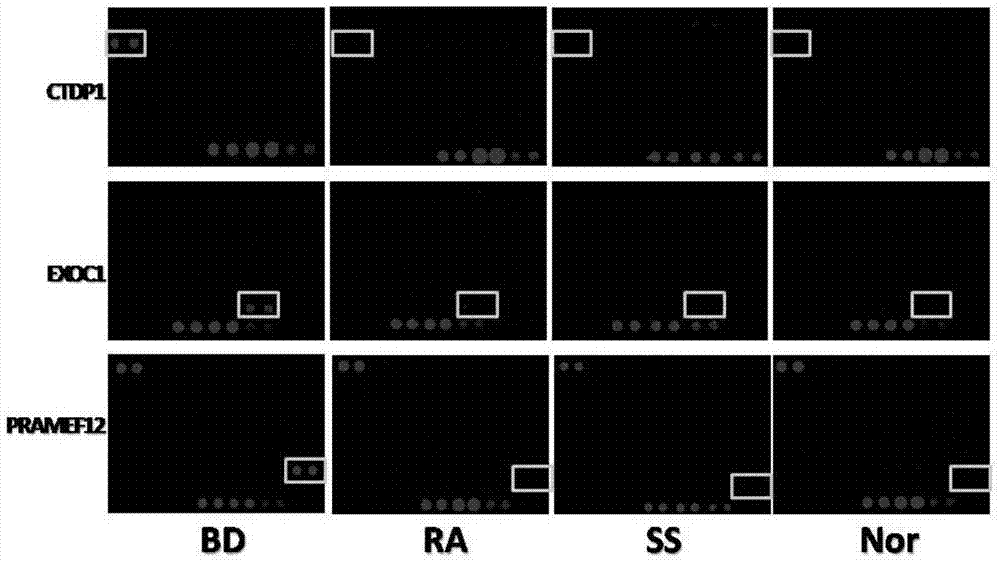
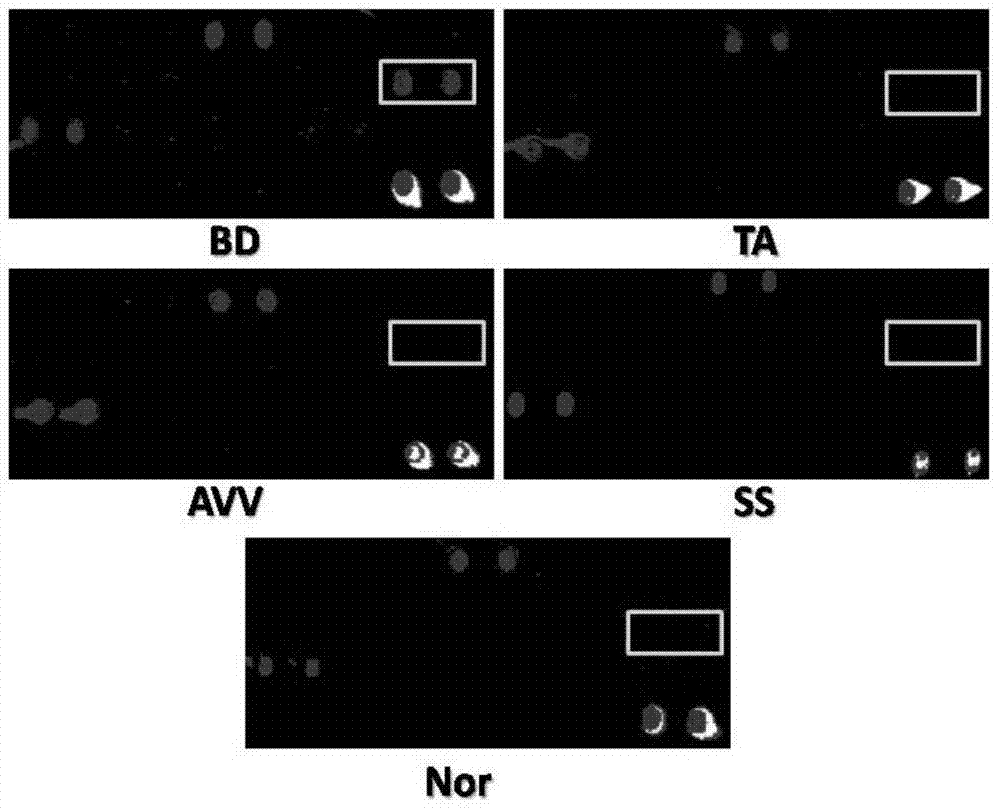
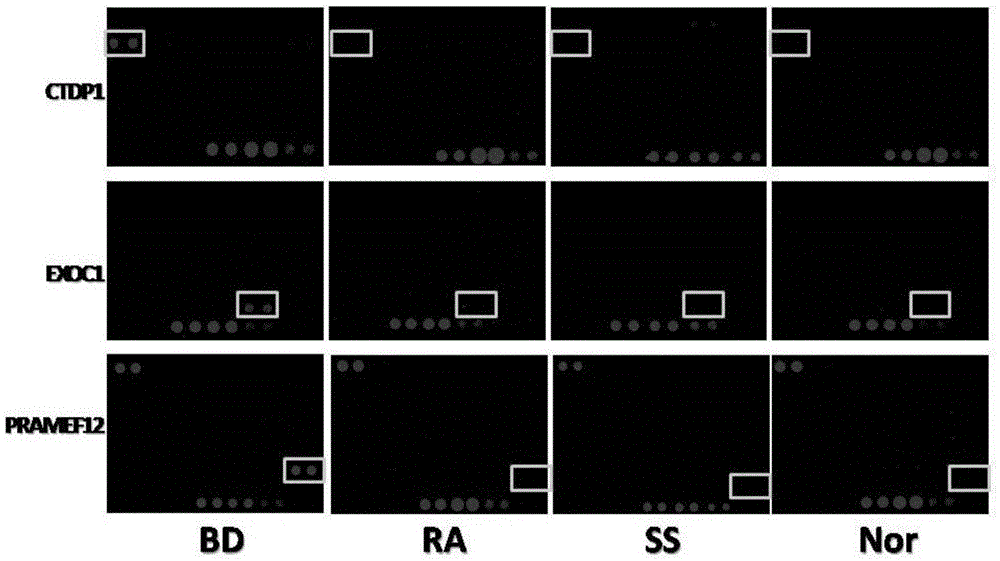
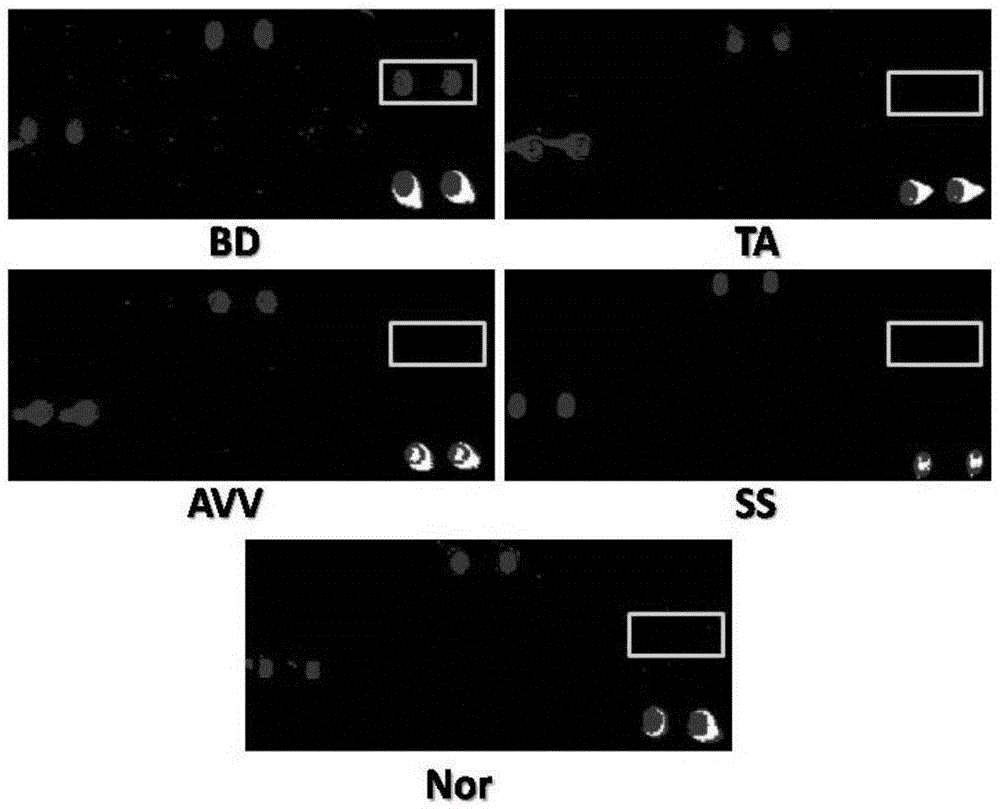
Biomarker for Behcet disease (BD) detection and use thereof
The invention provides a use of RNA polymerase II subunit A C-terminal domain phosphatase (CTDP1) or a fragment thereof in preparation of a reagent used for diagnosing Behcet disease (BD). The positive rate of the CTDP1 or the fragment thereof in a BD patient is remarkably higher than that of normal control, a Western blot verification result displays that an anti-CTDP1 antibody can be detected in the serum of the BD patient, which proves that the CTDP1 or the fragment thereof can be used for a detection marker of BD.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Microwave-driven rna polymerization by rna polymerases of caliciviruses
InactiveCN103119177APromote polymerizationOvercoming Degradation DefectsMicrobiological testing/measurementFermentationSingle strandPolynucleotide
The present invention relates to a method for polymerising a complementary RNA strand on a single-stranded polynucleotide template comprising the step of irradiating a composition containing said template and an RNA polymerase of a virus of the Caliciviridae family under RNA polymerisation conditions in the presence or absence of a primer hybridised to the template, with an effective amount of microwave energy. Further subject matter of the invention relates to a method for transferring one or more ribonucleotides to the 3' end of a single-stranded polynucleotide template comprising the step of irradiating a composition containing an RNA polymerase of a virus of the Caliciviridae family in the presence of rATP or rGTP or rUTP or rCTP or a modified or labelled analogue thereof with an effective amount of microwave energy.
Owner:RIBOXX
SiRNA expression vector and application thereof
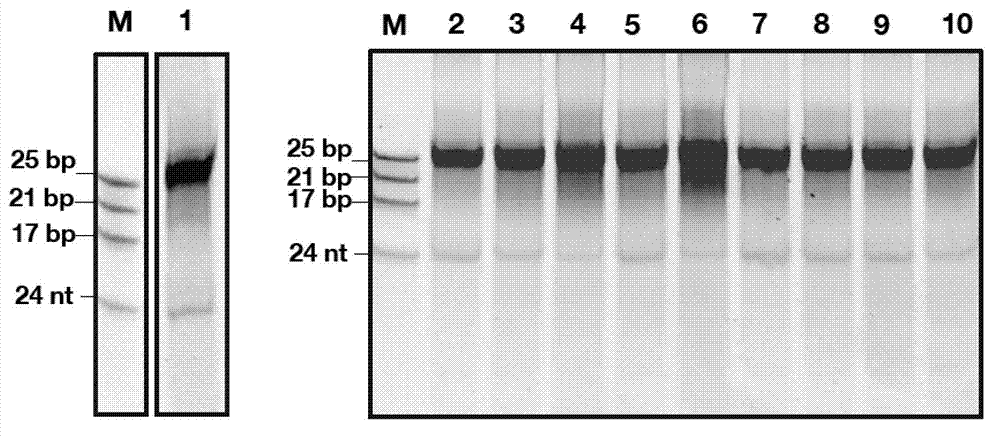
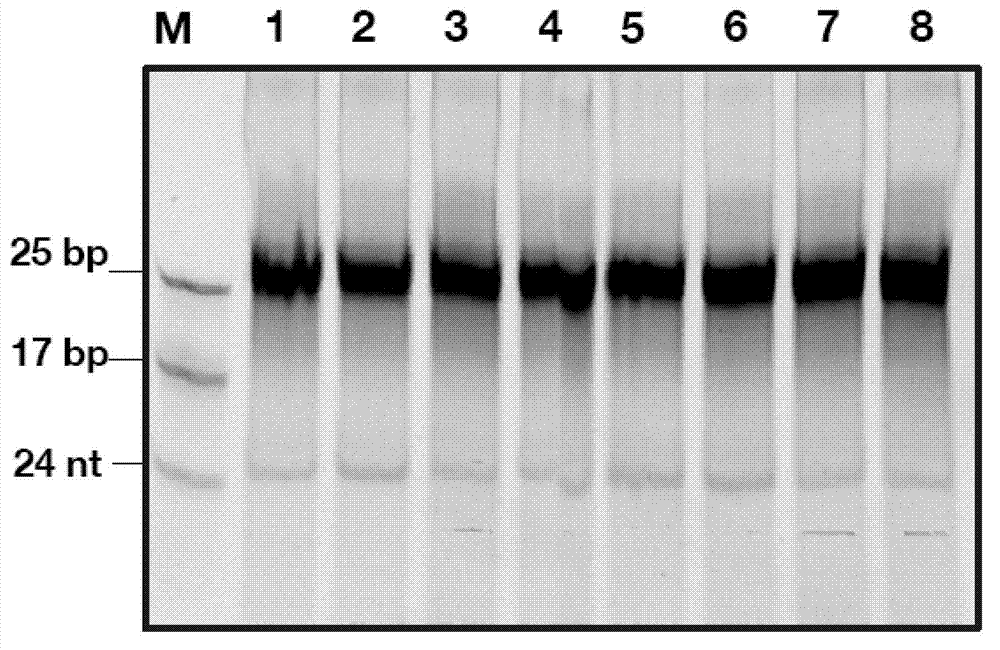
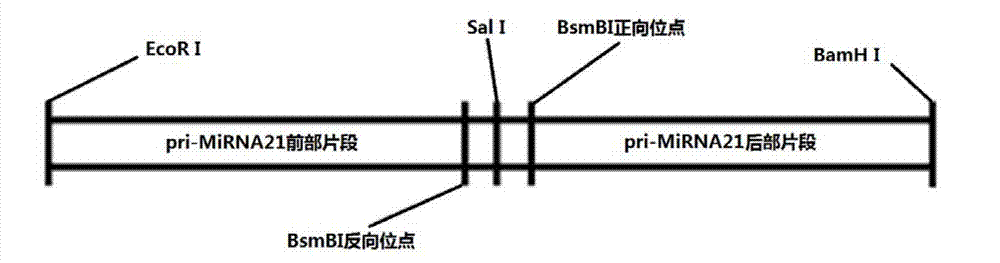
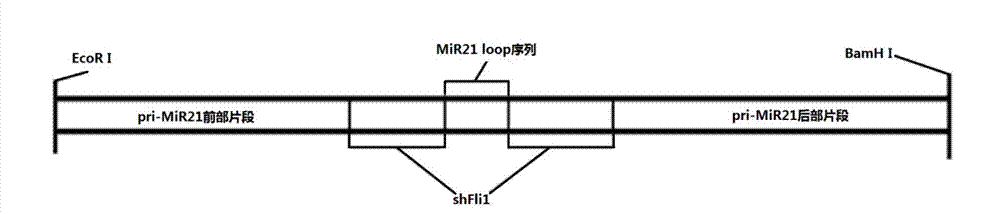
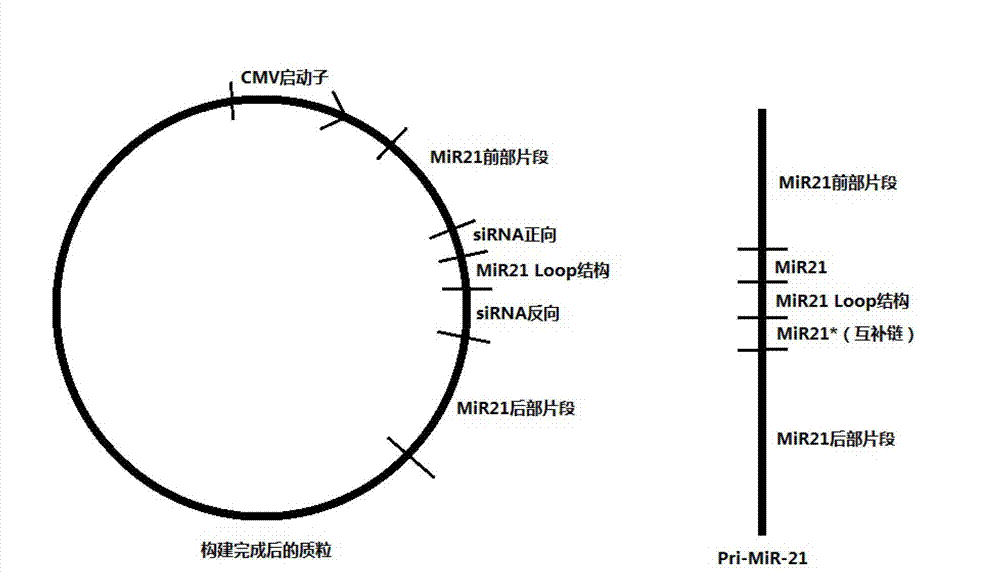
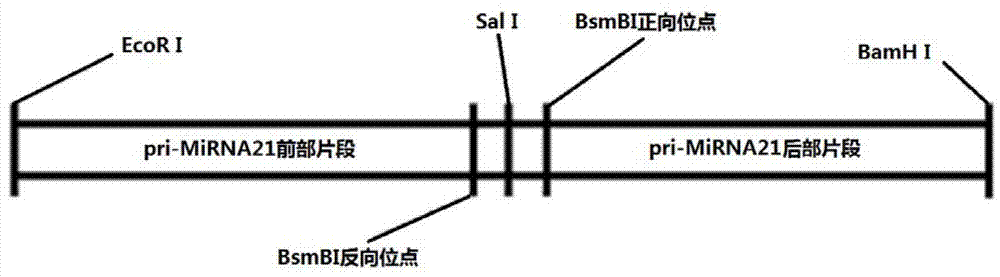
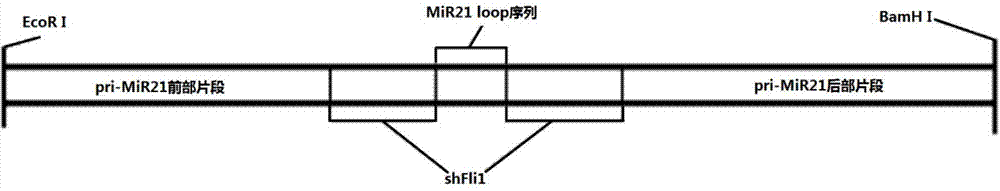
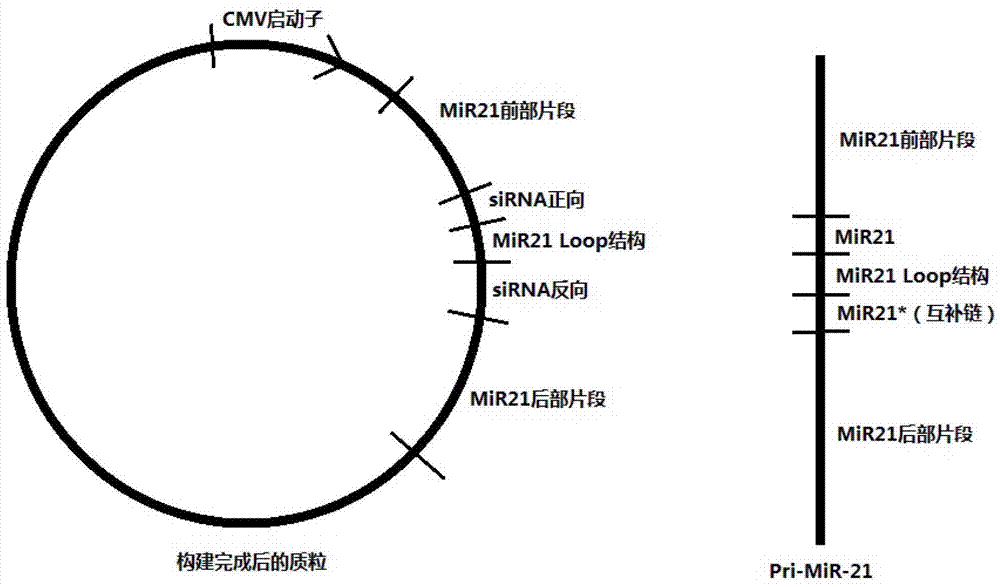
InactiveCN103205461AImprove knockdown efficiencyEfficient transfectionGenetic material ingredientsVector-based foreign material introductionRNA polymerase IIGenetics
The invention discloses an empty carrier for construction of a siRNA expression vector, and a recombinant vector for siRNA and application thereof. The empty carrier, from terminal 5' to terminal 3', comprises: 1) a CMV-derived RNA polymerase II dependent promoter sequence; 2) a pri-MiR21 front fragment sequence as represented by SEQ ID No. 1; 3) the sequence of a cleavage site of restriction endonuclease; 4) a pri-MiR21 rear fragment sequence as represented by SEQ ID No. 2; and 5) a terminator sequence. The recombinant vector for siRNA is obtained by inserting the sequence of siRNA sense sequence-MiR21 ring structure sequence-siRNA antisense sequence to the cleavage site of the restriction endonuclease. According to the invention, since an RNA Pol II promoter is employed, shRNA can be conveniently and effectively connected to the skeleton of pri-MiR21 and be stably expressed through the RNA Pol II promoter, high efficiency expression is realized, and efficiency of knockout and deletion of RNAi is improved.
Owner:重庆沃康生物科技有限公司
Vectors comprising novel regulatory elements
The invention relates to the field of recombinant DNA technology and, in particular, the development of vectors for the expression of recombinant proteins. Expression of heterologous genes in eukaryotic cells requires transcription by RNA polymerase II, which is driven by c / s-acting genetic elements known as promoters and enhancers. The invention provides promoters derived from the guinea pig cytomegalovirus early- immediate promoter / enhancer, useful for obtaining high levels of expression of recombinant proteins. Also disclosed are eukaryotic expression vectors comprising such promoters, which are capable of providing increased levels of expression over that obtainable from human or murine cytomegalovirus enhancer / promoter elements in many cell types.
Owner:EMD MILLIPORE CORP
Vector for lowering expression of cell-specific ndrg2 gene
InactiveCN101570762BDown-regulation effect is obviousEasy to observeVector-based foreign material introductionCell specificPreproinsulin
The invention discloses a vector for lowering expression of a cell-specific ndrg2 gene, which comprises an RNAi transgene vector, wherein the RNAi transgene vector is connected with a preproinsulin promoter; the downstream of the preproinsulin promoter is inserted with an miR access site; and the miR access site is connected with an miRNA sequence aiming at lowering the ndrg2 gene. The constructedRNAi transgene vector adopts an RNA polymerase II relied preproinsulin promoter, and a specific pancreatic islet beta cell of the preproinsulin promoter initiates the downstream miRNA sequence, initiates an RNA interference aiming at the ndrg2 gene and lowers the expression of the ndrg2 gene. The vector is applied to construction of animal models and researches on ndrg2 gene functions of the pancreatic islet beta cell.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
A biomarker for Behcet's disease detection and its application
The invention provides a use of RNA polymerase II subunit A C-terminal domain phosphatase (CTDP1) or a fragment thereof in preparation of a reagent used for diagnosing Behcet disease (BD). The positive rate of the CTDP1 or the fragment thereof in a BD patient is remarkably higher than that of normal control, a Western blot verification result displays that an anti-CTDP1 antibody can be detected in the serum of the BD patient, which proves that the CTDP1 or the fragment thereof can be used for a detection marker of BD.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
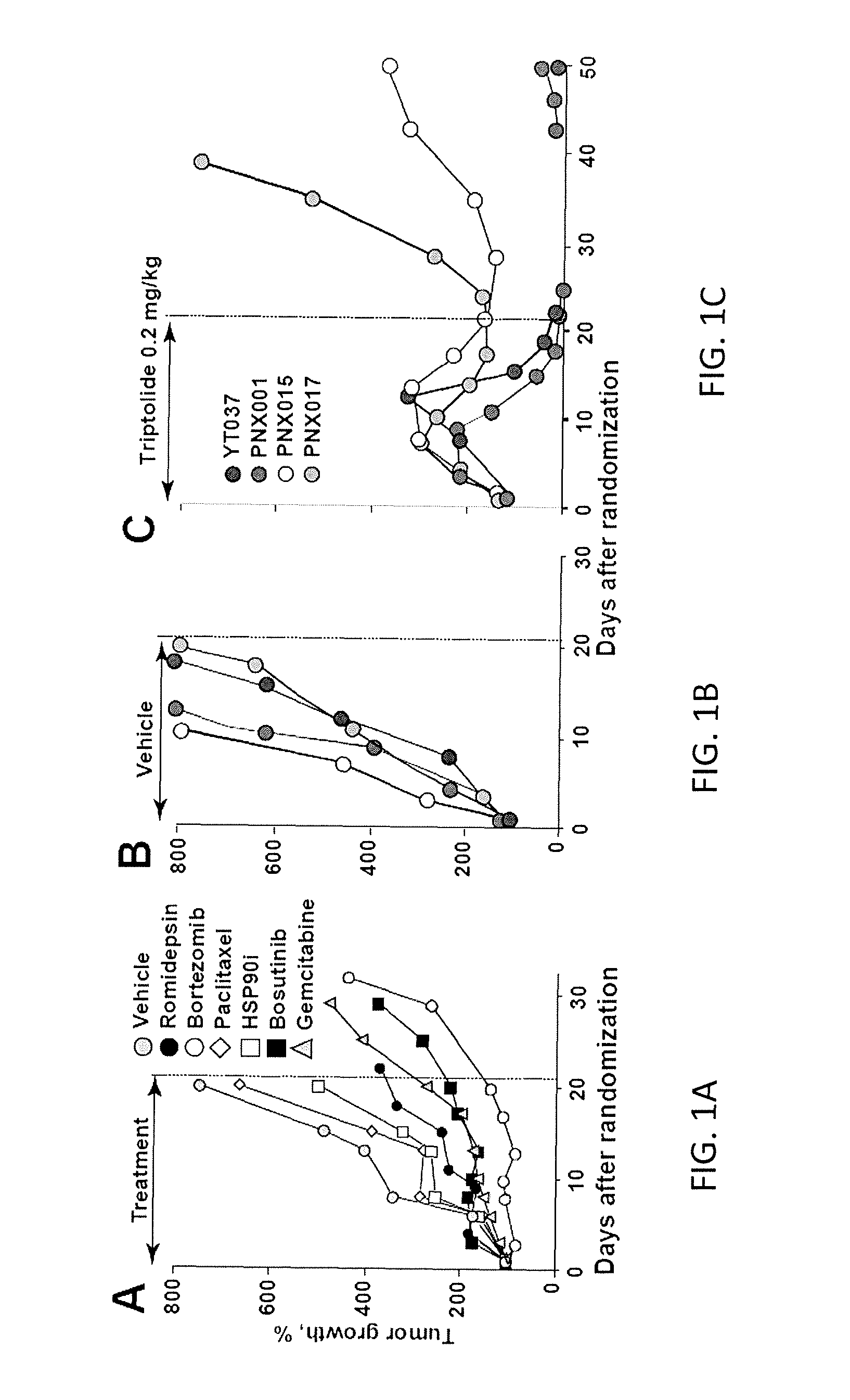
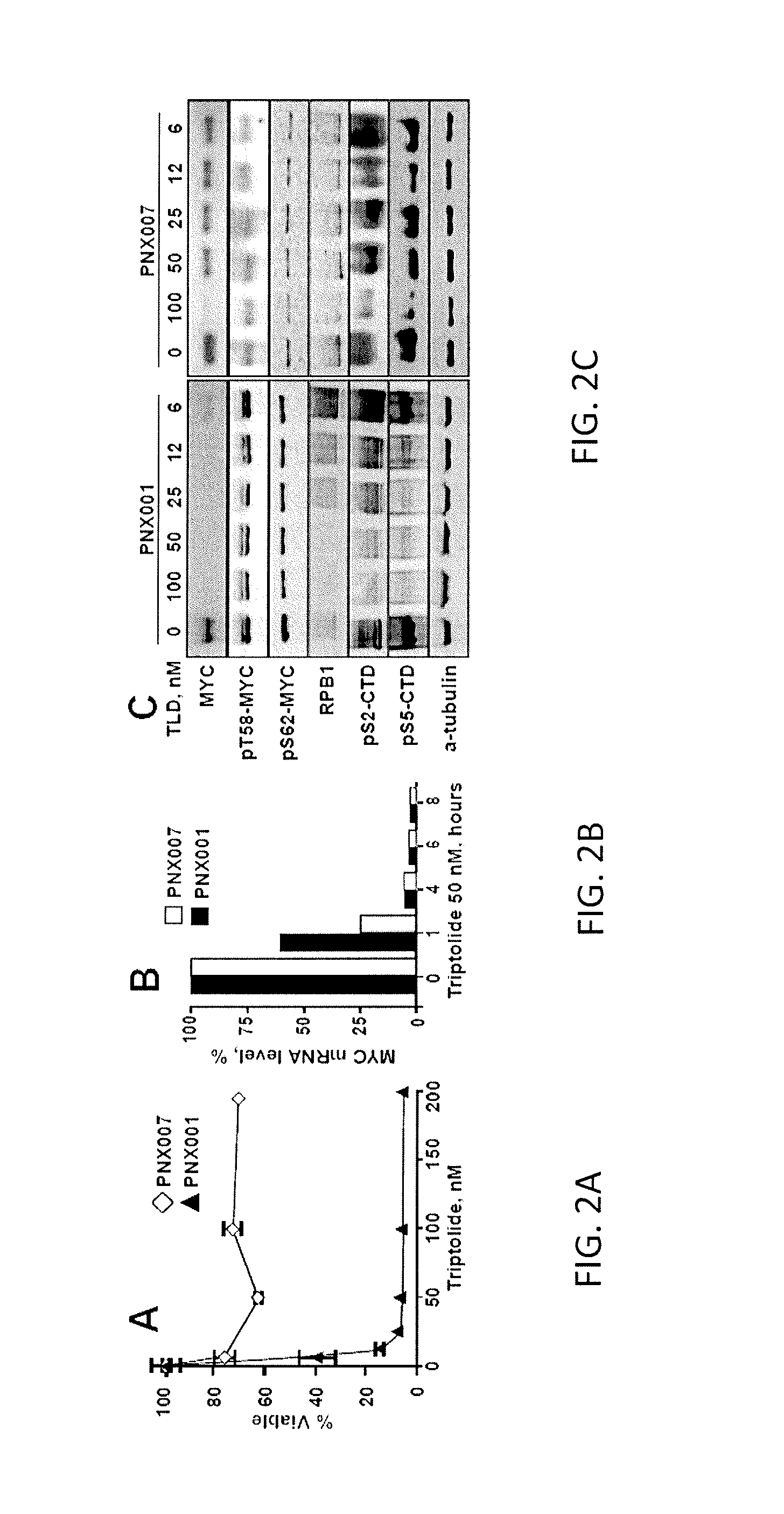
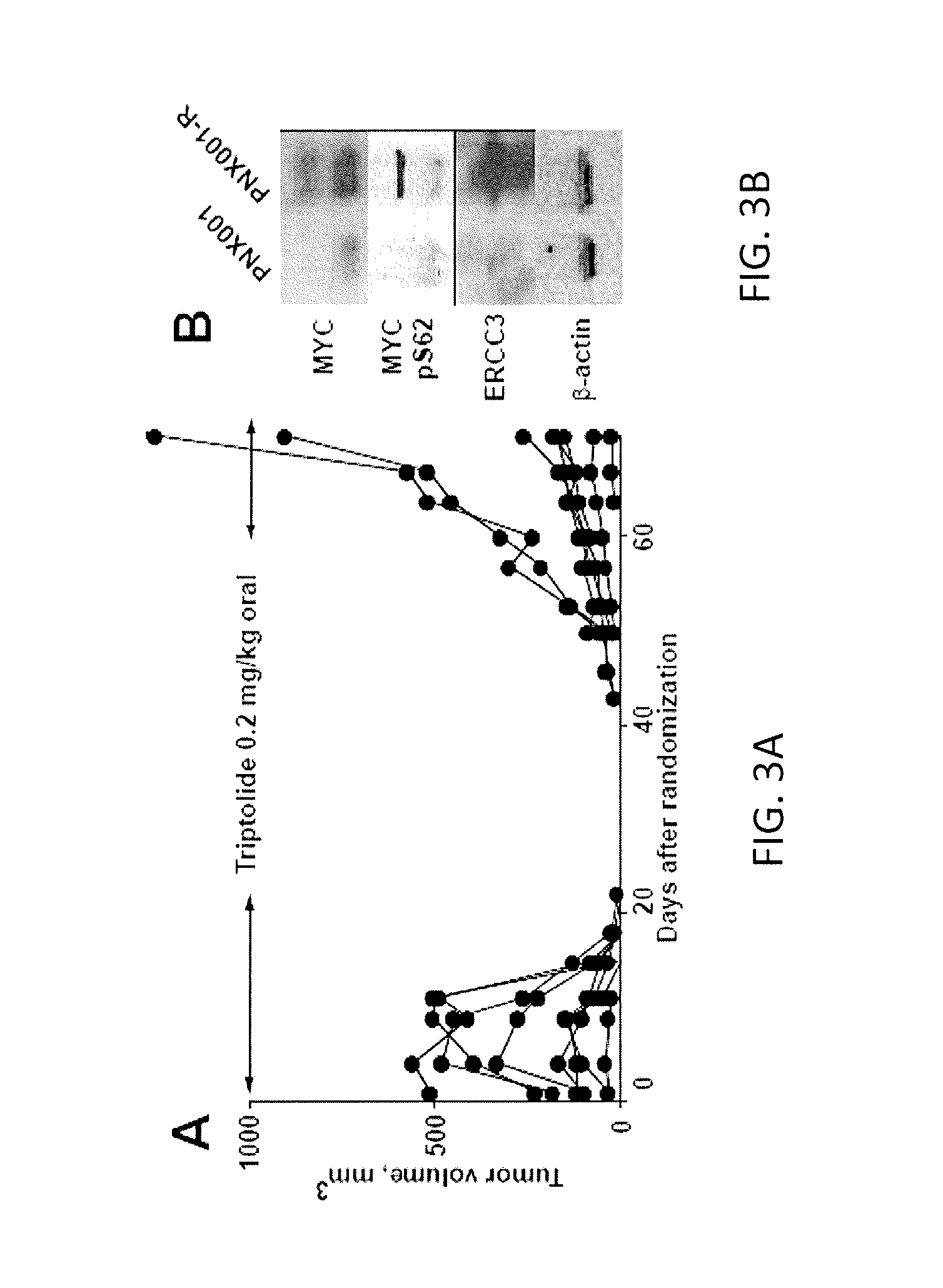
C-MYC as a Biomarker for Tumor Sensitivity to Treatment with RNA Polymerase II Inhibition
ActiveUS20160354339A1Inhibit biological activityInhibit biologic activityOrganic active ingredientsOrganic chemistryAbnormal tissue growthRNA polymerase II
Systems and methods for determining whether a cancer patient may respond to inhibition of RNA polymerase II as a treatment for the patient are provided. Inhibition of RNA polymerase II may be by way of chemotherapy with an agent such as triptolide, an analog of triptolide, or a prodrug form of triptolide. The cancer patient may be a pancreatic cancer patient, an ovarian cancer patient, a gastric cancer patient, or an esophageal cancer patient. The patient may have any cancer in which c-Myc is over-expressed or over-amplified.
Owner:NEXUS PHARM INC +1
Internal reference gene of Fengdan and its special primers and application under drought stress
ActiveCN111733168BImprove stabilityImprove reliabilityMicrobiological testing/measurementOxidoreductasesInitiation factorTranslation Initiation Factor
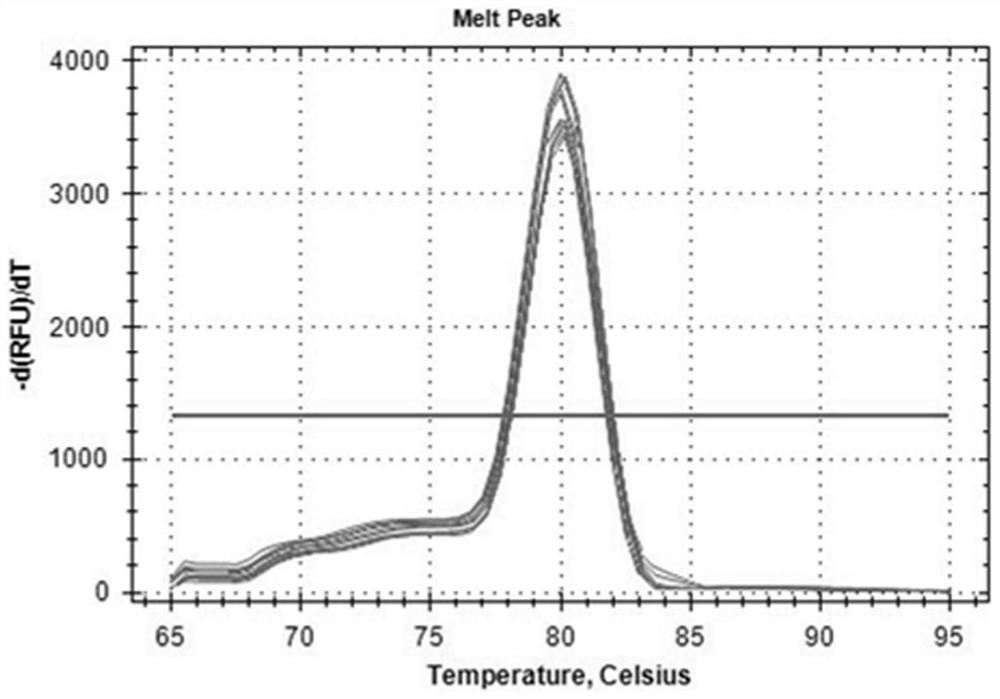
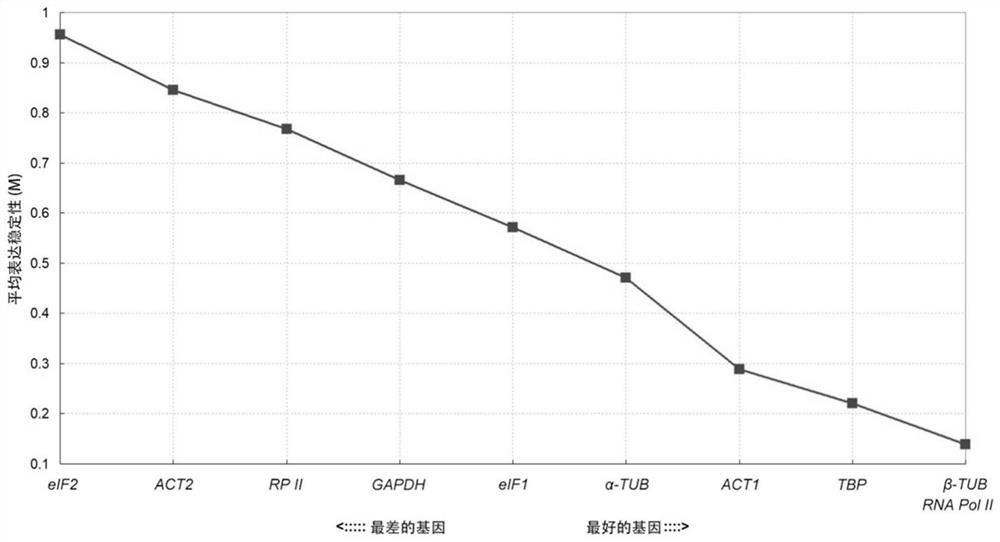
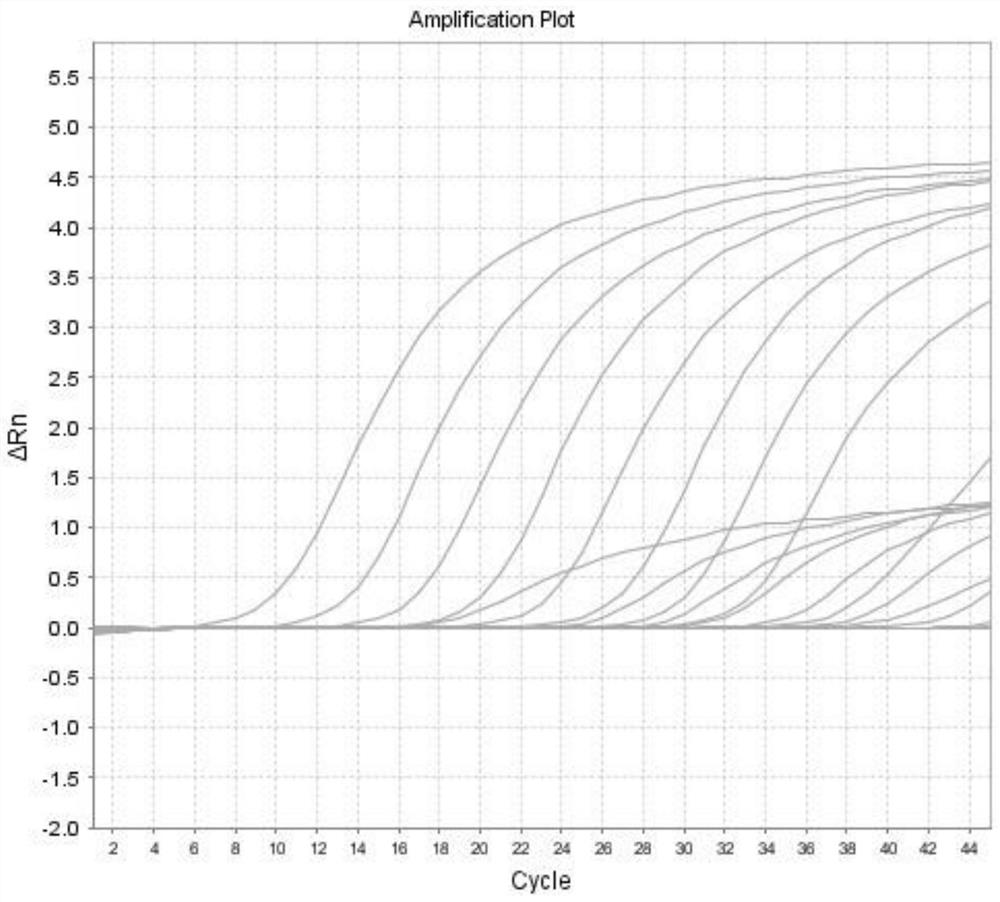
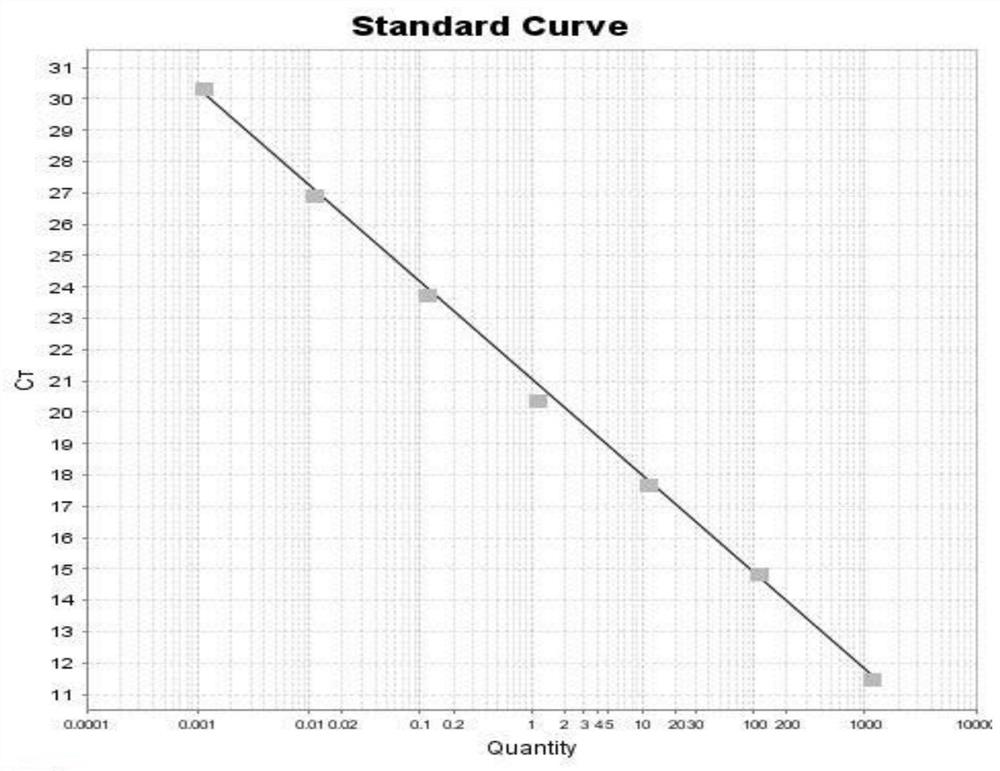
The present invention relates to an internal reference gene of Fengdan under drought stress, its special primers and its application. The internal reference genes are TATA box binding protein TBP gene, actin ACT1 gene, actin ACT2 gene, glyceraldehyde-3-phosphate dehydrogenase GAPDH gene , eukaryotic translation initiation factor eIF1 gene, eukaryotic translation initiation factor eIF2 gene, tubulin α-TUB gene, tubulin β-TUB gene, RNA polymerase II RNA Pol II gene or RNA polymerase II Transcription factor RP II gene; the nucleotide sequence of the above gene is shown in the sequence list. The purpose of the present invention is to provide a gene stably expressed in the gene expression profile of Fengdan under drought stress, which is used as an internal reference gene for drought stress of Fengdan.
Owner:YANGZHOU UNIV
Real-time fluorescent quantitative PCR primer probe set, kit and detection method for detecting African swine fever virus
PendingCN113801962ARealize quantitative detectionReduce the possibilityMicrobiological testing/measurementMicroorganism based processesFluoProbesVirus detection
The invention discloses a real-time fluorescent quantitative PCR primer probe set, a kit and a detection method for detecting African swine fever virus, and relates to the technical field of virus detection. The real-time fluorescent quantitative PCR primer probe set comprises a primer and a probe for detecting an African swine fever virus B646L gene and a primer and a probe for detecting a swine RNA polymerase II gene. The invention also provides the real-time fluorescent quantitative PCR kit and the detection method for detecting the African swine fever virus. According to the kit, a fluorescent PCR technology is adopted, and quantitative detection of the African swine fever virus is achieved through a TaqMan probe marked by a fluorescent reporter group; in addition, a reaction system also comprises a primer for detecting a pig RNA polymerase II gene conserved region and a fluorescent probe marked by different fluorescent reporter groups, which are used as internal quality control and are used for monitoring whether sample collection is standard or not and whether nucleic acid extraction and PCR amplification are successful or not.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Rhizopus oryzae variant NC-6 for producing L-lactic acid with high yield and high optical purity and application of Rhizopus oryzae variant NC-6
ActiveCN111621426AHigh and stable yieldLow costFungiMicroorganism based processesSequence analysisRhizopus oryzae
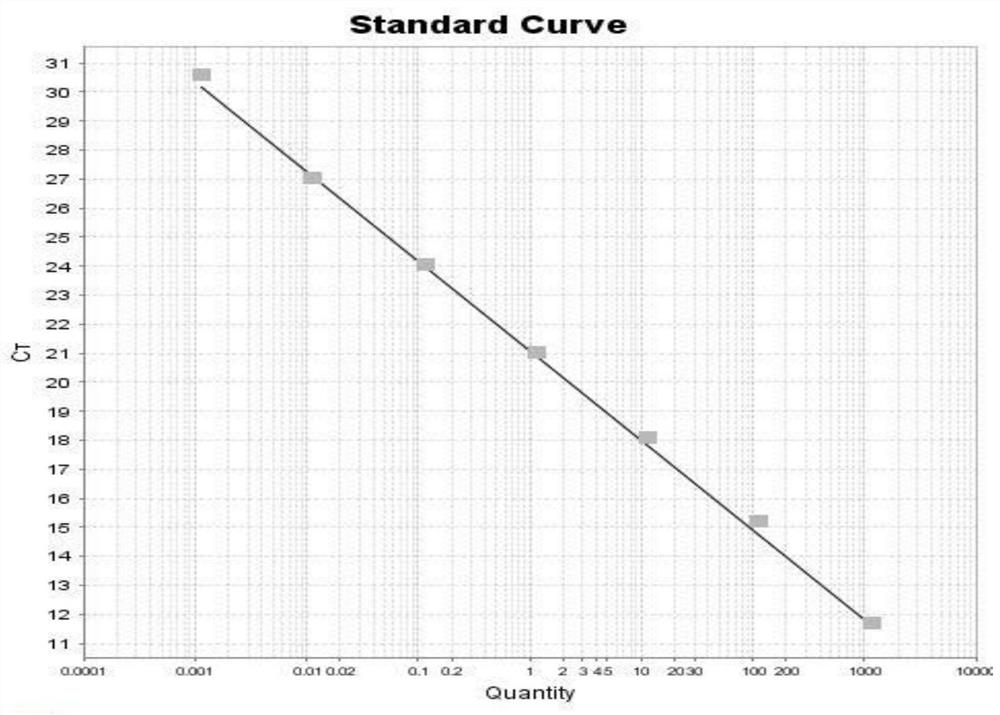
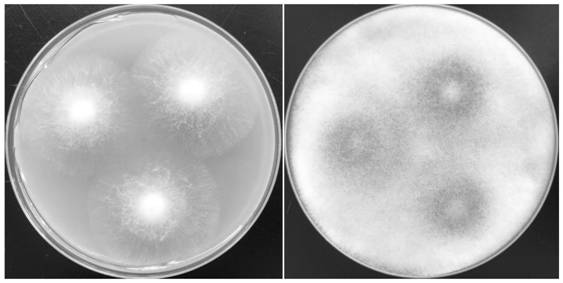
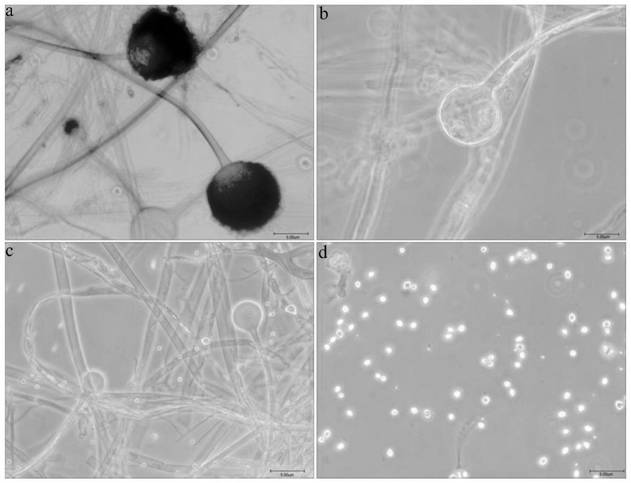
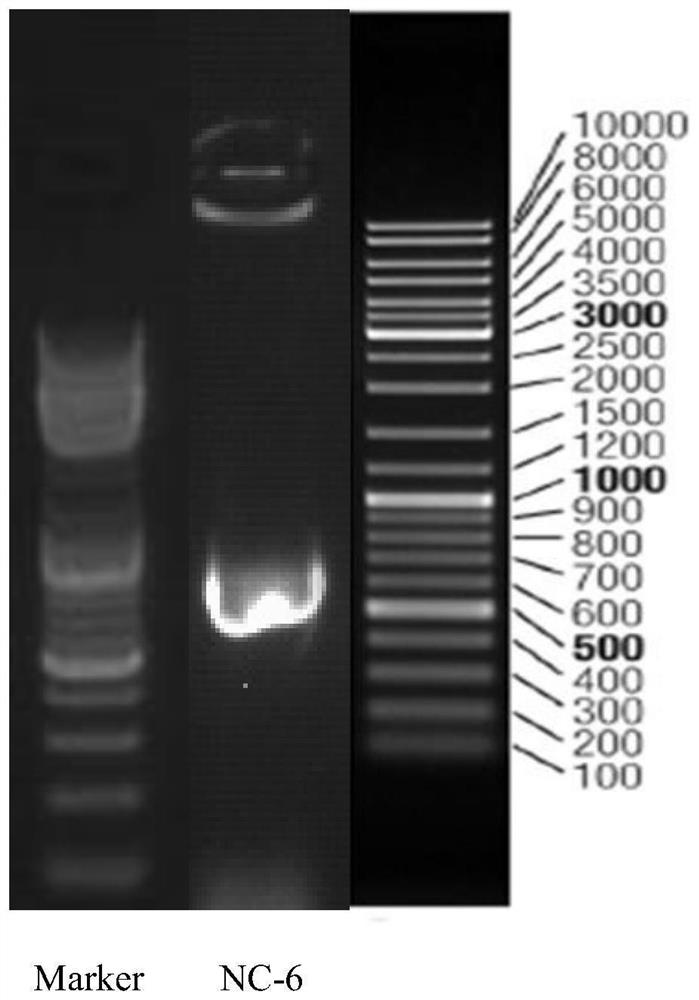
The invention discloses a Rhizopus oryzae variant NC-6 for producing L-lactic acid with high yield and high optical purity. The Rhizopus oryzae variant NC-6 was preserved in China General Microbiological Culture Collection Center (CGMCC) on April 20th, 2020, the preservation address is No. 3, No.1 Yard, Beichenxi Road, Chaoyang District, Beijing City, the Rhizopus oryzae variant NC-6 is classifiedand named as Rhizopus oryzae, and the preservation number is CGMCC NO. 19620. The high-yield strain for producing the L-lactic acid with high yield and high optical purity is specifically identifiedas a new Rhizopus oryzae variant by performing morphological analysis, ITS rDNA sequence analysis, whole genome re-sequencing analysis and comparative analysis on sequence similarity Blast of proteincoding genes actin, EF-1 alpha (translation elongation factor 1-alpha) and RPB2 (the largest subunit of the RNA polymerase II). The strain is cultured by shake flask fermentation, and it discovers that the L-lactic acid yield of the strain reaches 78.00 g / L, the sugar conversion rate reaches 73.58%, and the optical purity is 100%; and the strain is cultured by amplification in a 200 L fermentationtank, the L-lactic acid yield of the strain reaches 86.07 g / L, the sugar conversion rate is 83.21%, the optical purity is 100%, and the yield is stable. The strain has wide industrial application prospect.
Owner:江西科院生物新材料有限公司
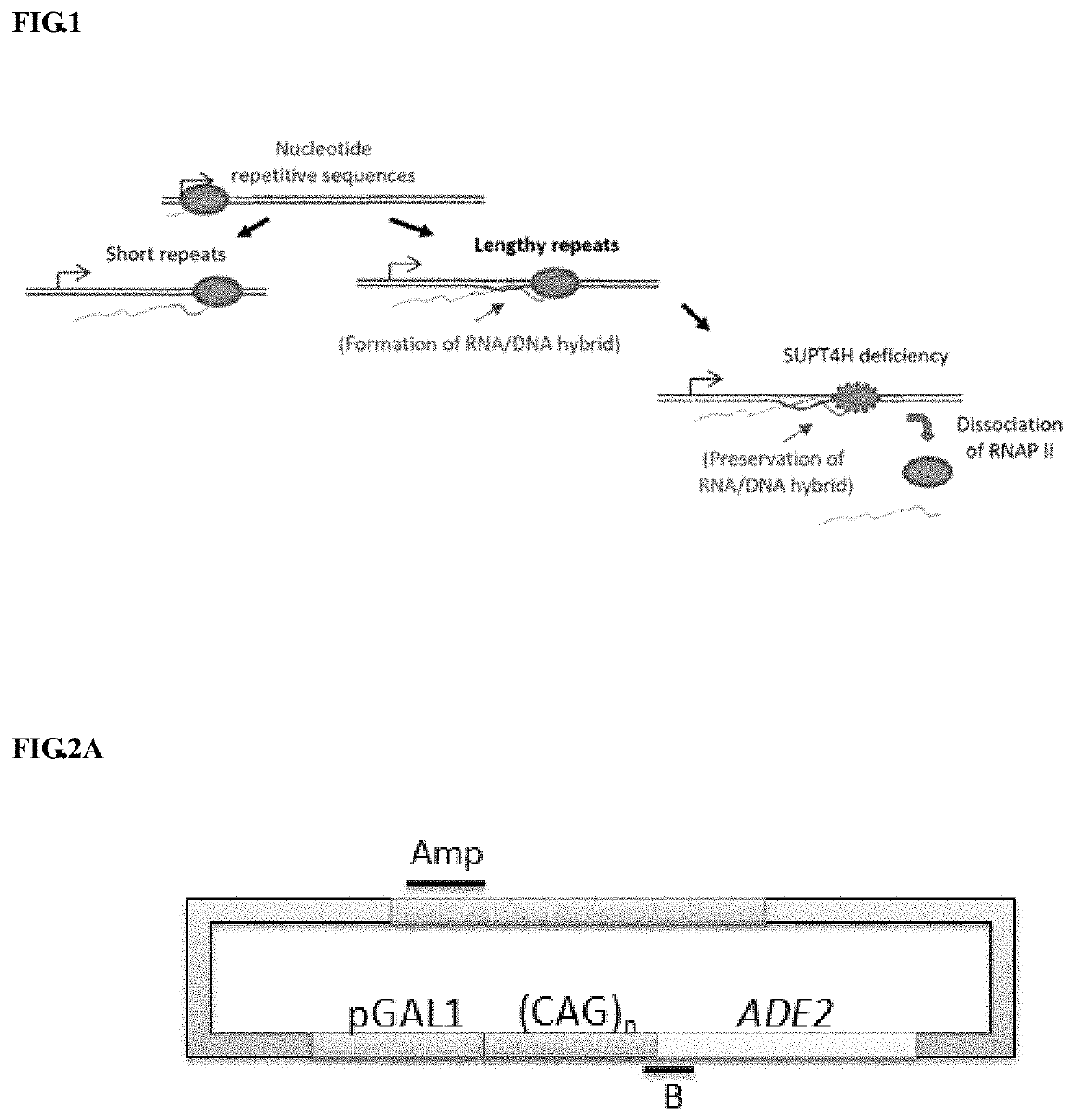
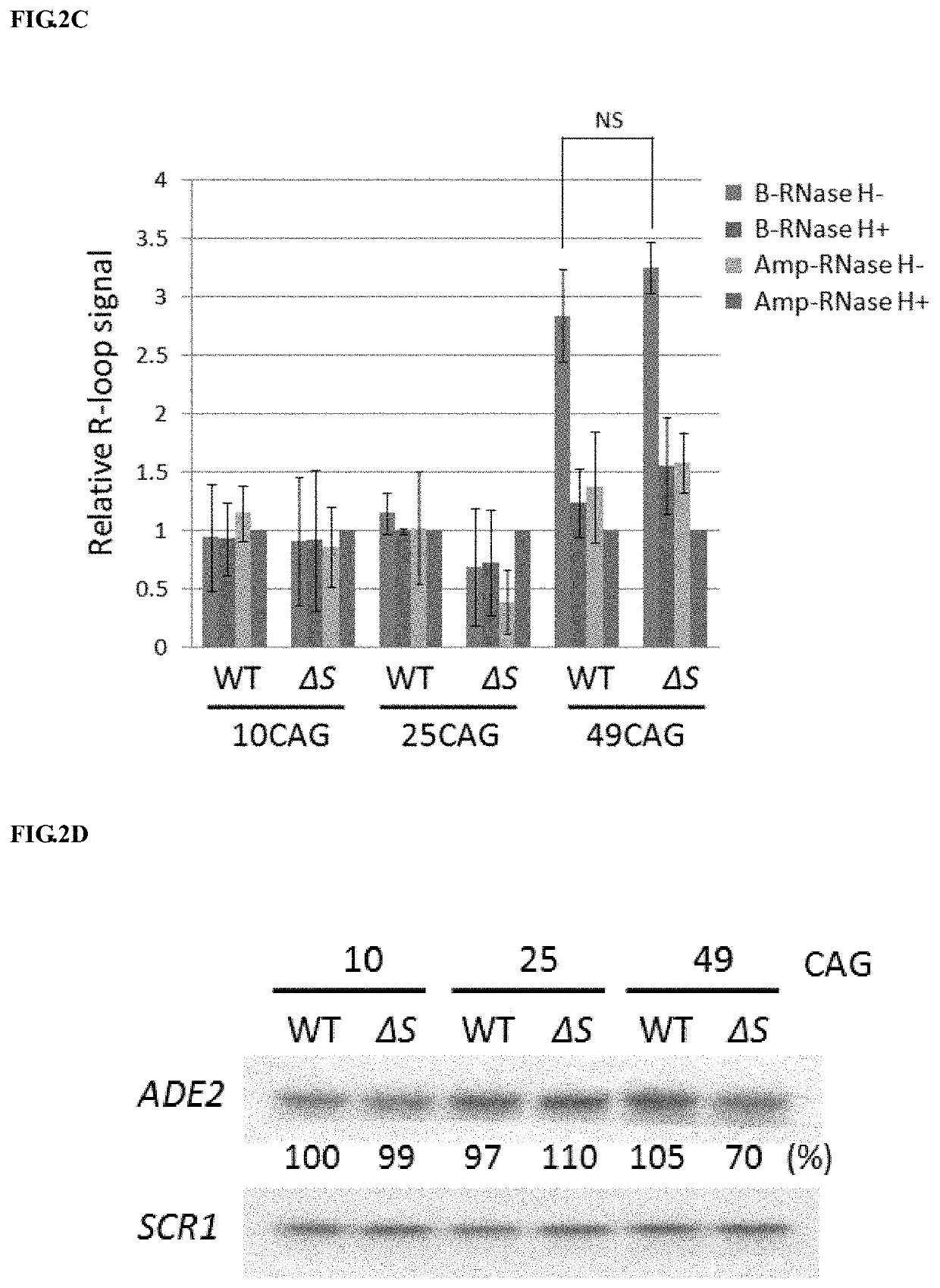
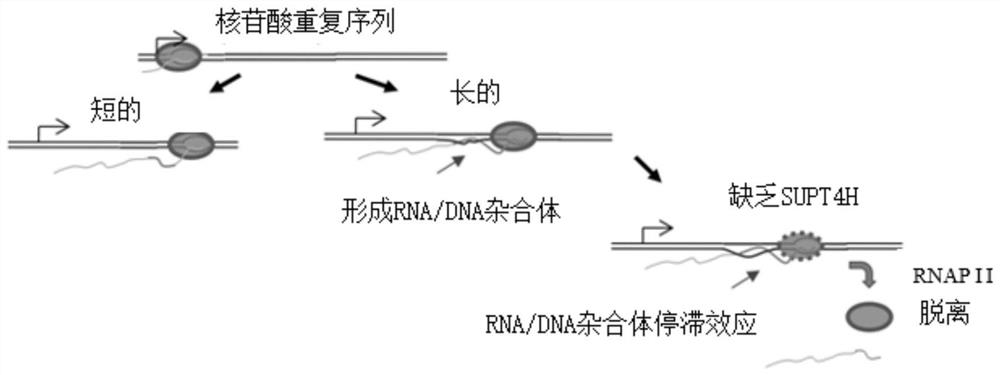
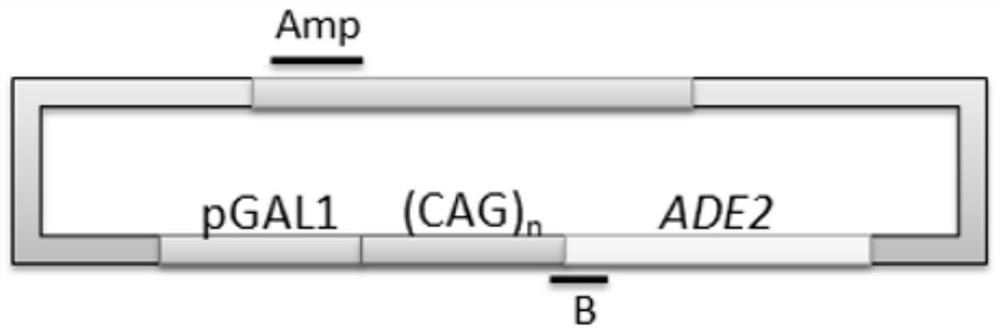
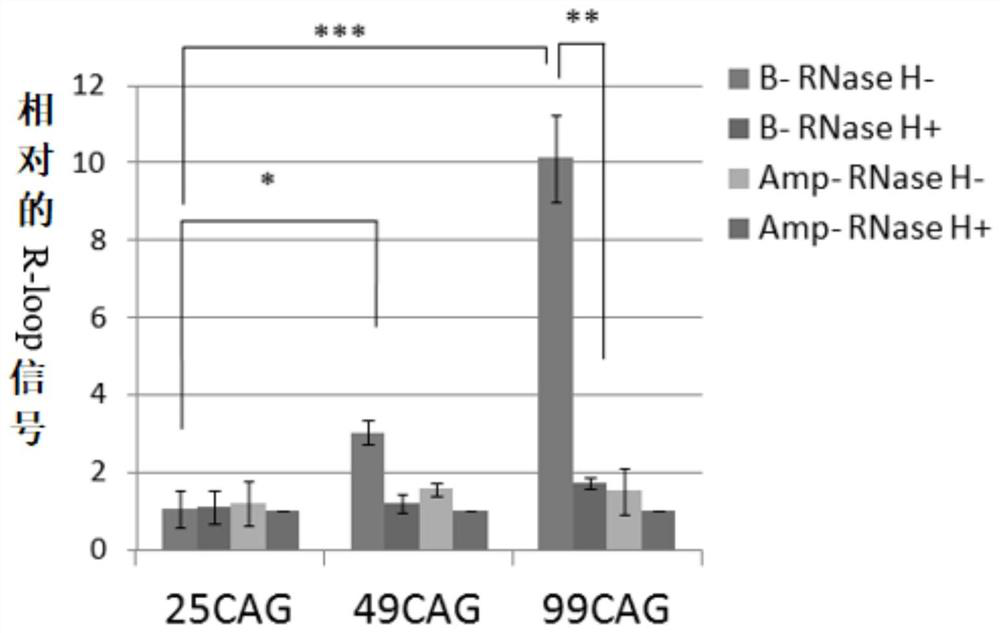
Method to enhance the transcription regulation of SUPT4H on genes containing repetitive nucleotide sequences
PendingUS20210040479A1Reduce expressionOrganic active ingredientsNervous disorderNucleotideRNA polymerase II
The present invention provides a method of modulating the expression of a gene containing expanded nucleotide repeats in a cell, comprising: inhibiting the biological activity of SPT4 or SUPT4H; and regulating the formation of R-loops. The inhibition step can effectively reduce the expression of the gene containing the expanded nucleotide repeats and the regulatory step can further enhance the inhibition step. The inhibition step and the regulation step are for the purpose of regulating gene expression by interfering the capacity of RNA polymerase II transcribing over a DNA template with lengthy nucleotide repeats.
Owner:NATIONAL YANG MING UNIVERSITY
Method to enhance the transcription regulation of SUPT4H on genes containing repetitive nucleotide sequences
Provided is a method of modulating the expression of a gene containing expanded nucleotide repeats in a cell, comprising: inhibiting the biological activity of SPT4 or SUPT4H; and regulating the formation of R-loops. The inhibition step can effectively reduce the expression of the gene containing the expanded nucleotide repeats and the regulatory step can further enhance the inhibition step. The inhibition step and the regulation step are for the purpose of regulating gene expression by interfering the capacity of RNA polymerase II transcribing over a DNA template with lengthy nucleotide repeats.
Owner:TAIWAN STRATEGICS INTPROP CO LTD
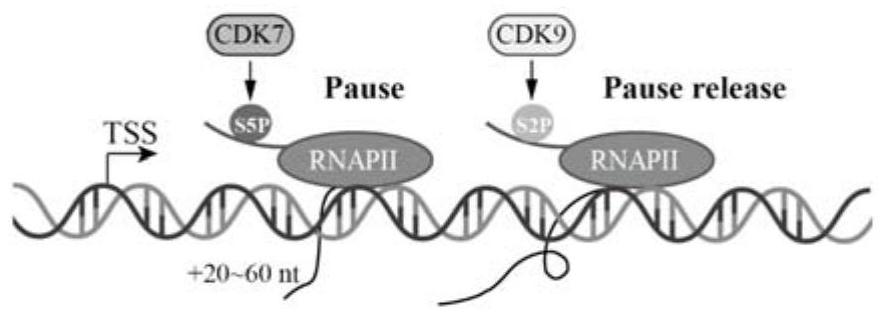
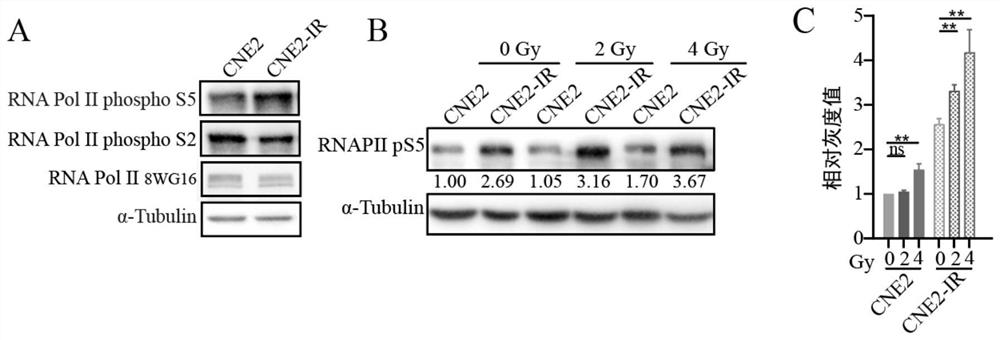
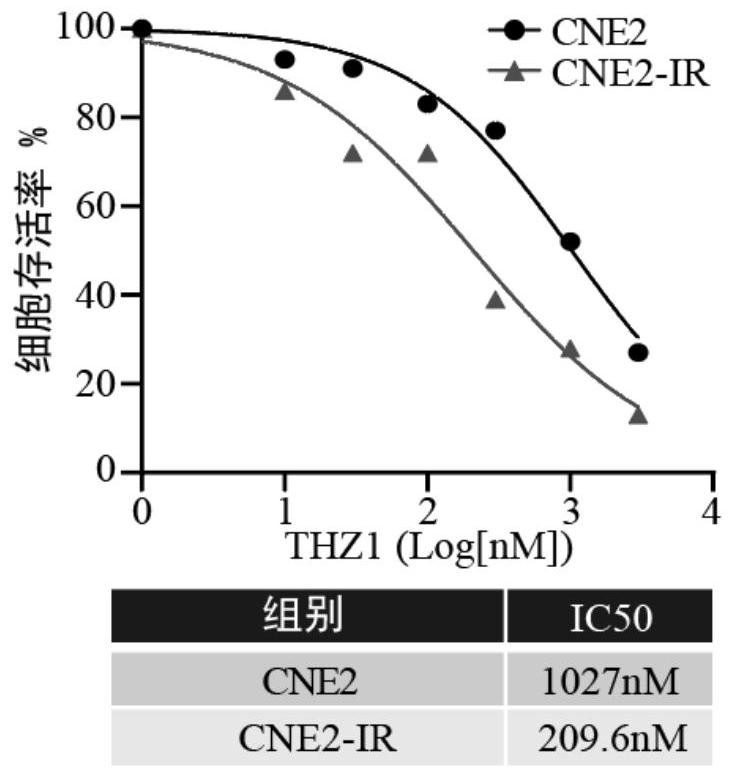
Application of CDK7 inhibitor THZ1 in nasopharynx cancer radiotherapy resistance treatment
PendingCN114344305AGrowth inhibitionImprove radiosensitivityOrganic active ingredientsAntineoplastic agentsParanasal Sinus CarcinomaPhosphorylation
The invention relates to the technical field of biology, in particular to application of a CDK7 inhibitor THZ1 in nasopharyngeal carcinoma radiotherapy resistance treatment. According to the invention, the difference of phosphorylation levels of RNA polymerase II C-terminal repetitive domain S5 in nasopharyngeal carcinoma radiotherapy resistance and radiotherapy sensitive cells and after radiotherapy irradiation is carried out on the nasopharyngeal carcinoma radiotherapy resistance and radiotherapy sensitive cells is firstly explored. An inhibitor THZ1 of a CDK7 target spot is adopted to detect nasopharyngeal carcinoma radiotherapy resistance and drug sensitivity of sensitive cells. THZ1 and radiotherapy are combined to act on radiotherapy-resistant cells, and whether THZ1 can increase radiotherapy sensitivity or not is explored. THZ1 is applied to a radiotherapy-resistant mouse model, and the treatment effect of THZ1 on radiotherapy-resistant tumors is explored. The CDK7 inhibitor THZ1 provided by the invention can effectively inhibit the growth of nasopharyngeal carcinoma radiotherapy resistant tumors in an in-vitro level cell experiment and an in-vivo level nude mouse subcutaneous tumor formation experiment. From the cellular level and the animal level, when the THZ1 is combined with the radiotherapy, the radiotherapy sensitivity can be increased, which indicates that the THZ1 is feasible to treat the nasopharyngeal carcinoma radiotherapy resistance.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
SiRNA expression vector and application thereof
InactiveCN103205461BImprove knockdown efficiencyEfficient transfectionGenetic material ingredientsGene therapyRNA polymerase IIGenetics
The invention discloses an empty carrier for construction of a siRNA expression vector, and a recombinant vector for siRNA and application thereof. The empty carrier, from terminal 5' to terminal 3', comprises: 1) a CMV-derived RNA polymerase II dependent promoter sequence; 2) a pri-MiR21 front fragment sequence as represented by SEQ ID No. 1; 3) the sequence of a cleavage site of restriction endonuclease; 4) a pri-MiR21 rear fragment sequence as represented by SEQ ID No. 2; and 5) a terminator sequence. The recombinant vector for siRNA is obtained by inserting the sequence of siRNA sense sequence-MiR21 ring structure sequence-siRNA antisense sequence to the cleavage site of the restriction endonuclease. According to the invention, since an RNA Pol II promoter is employed, shRNA can be conveniently and effectively connected to the skeleton of pri-MiR21 and be stably expressed through the RNA Pol II promoter, high efficiency expression is realized, and efficiency of knockout and deletion of RNAi is improved.
Owner:重庆沃康生物科技有限公司
Reporter for RNA Polymerase II Termination
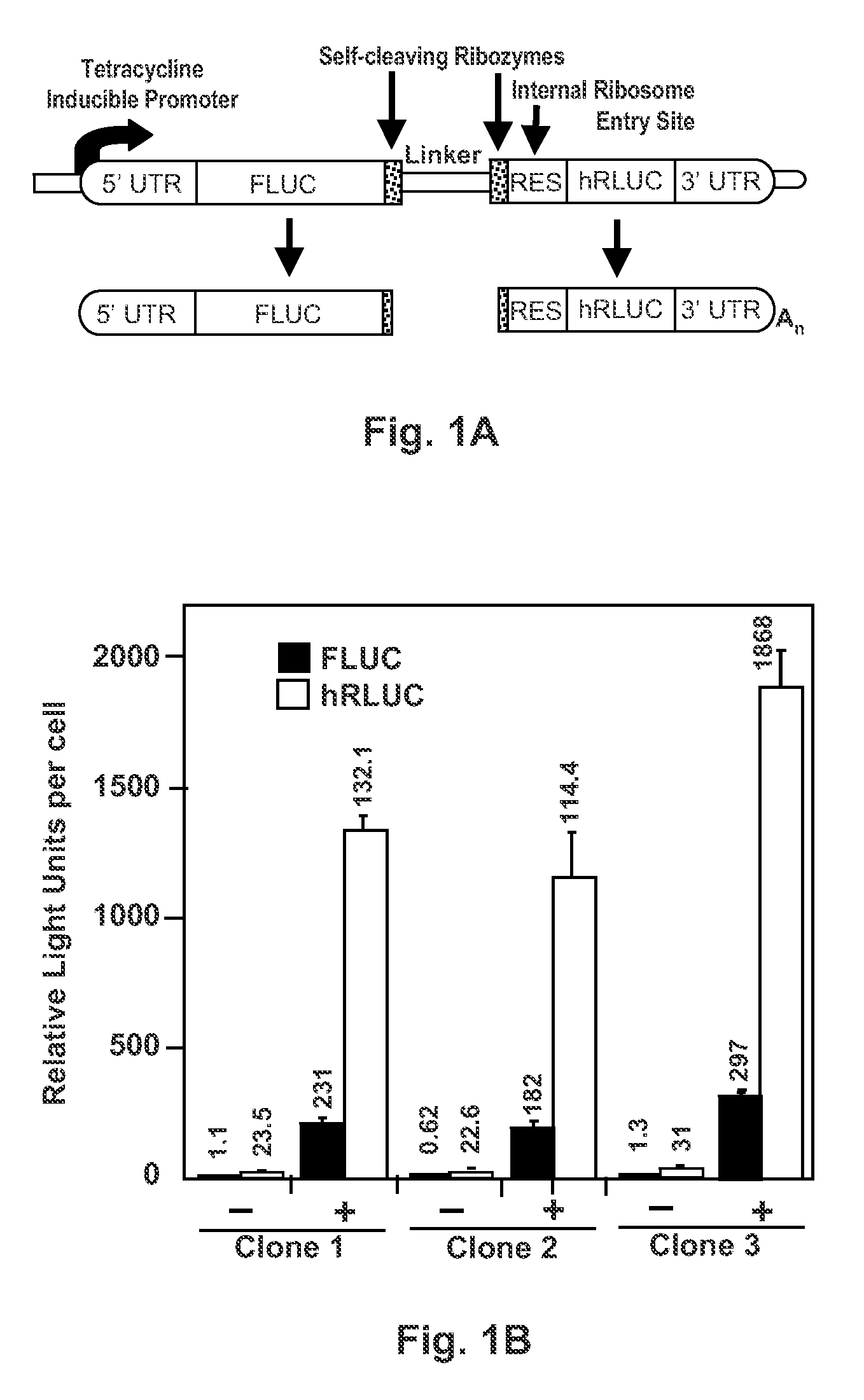
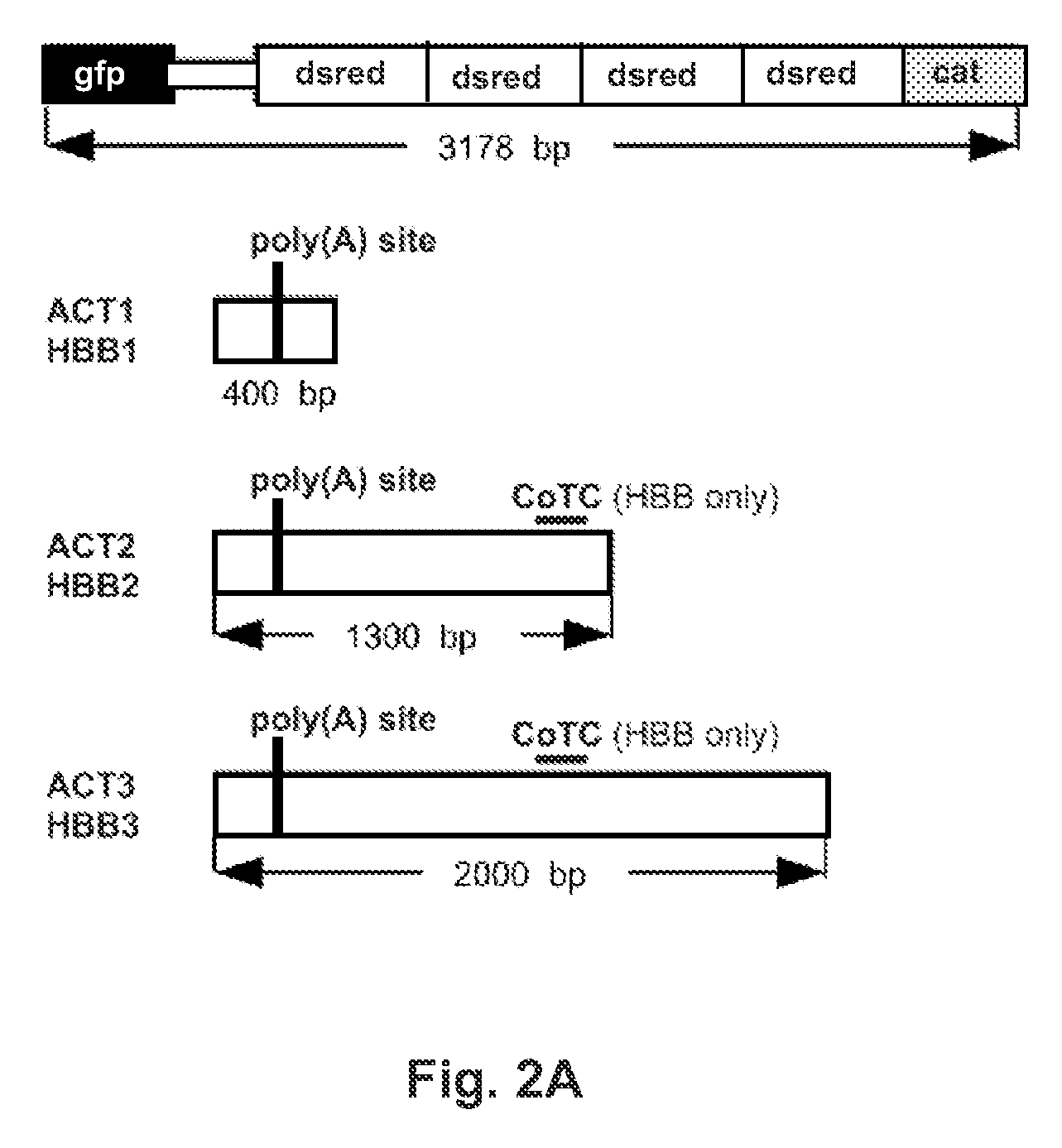
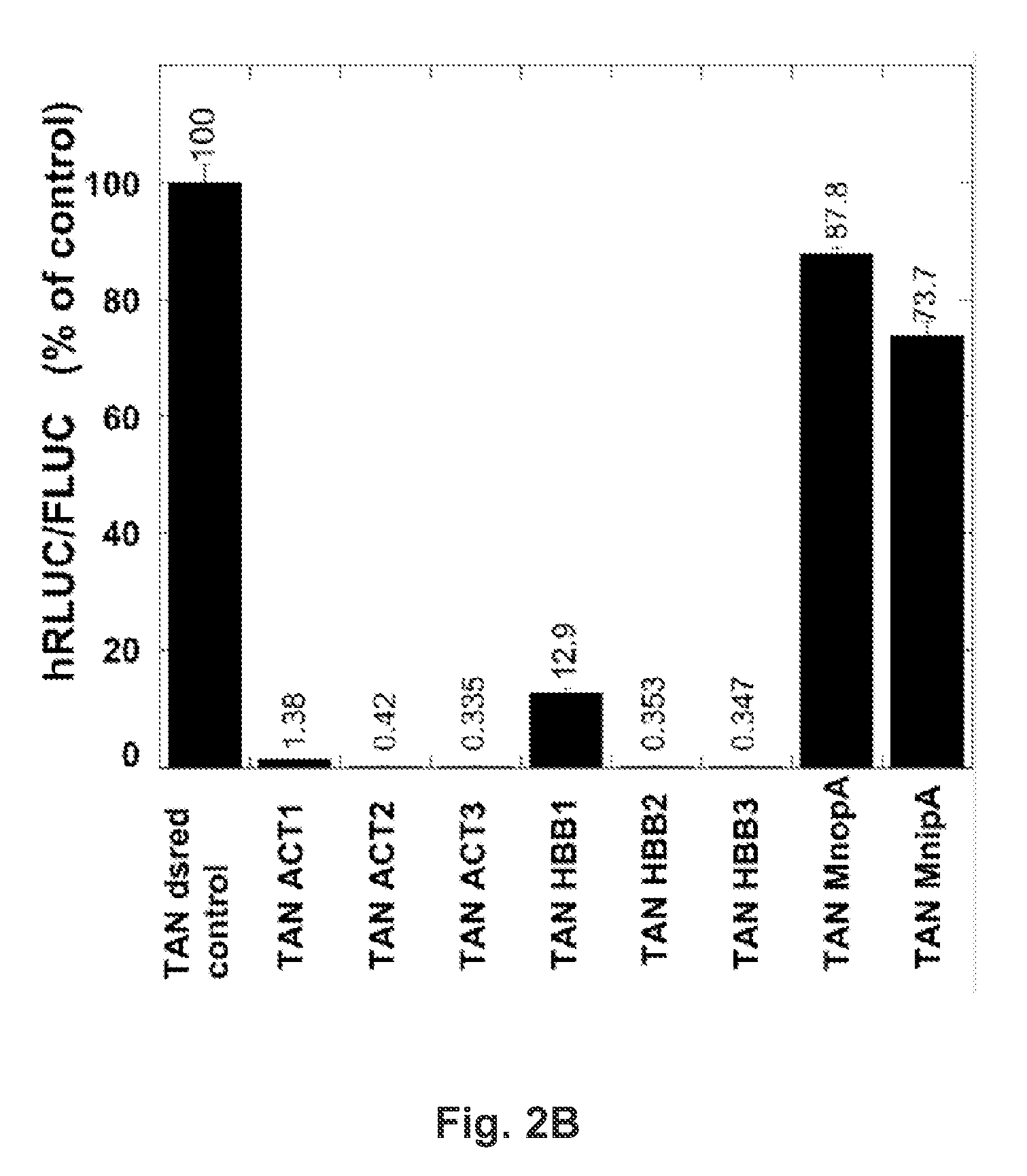
InactiveUS20130244905A1Short activityReduced effectivenessMicrobiological testing/measurementLibrary screeningRNA polymerase IITest sequence
A “tandem” reporter construct is disclosed that is capable of assaying RNA transcription termination. The ratio of expression between an upstream reporter and a downstream reporter as compared to the ratio observed for a control construct provides a measure of the relative rate of successful elongation through the intervening sequence. In one embodiment, two self-cleaving ribozymes separate the reporters from a test sequence between them.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
CRISPR/Cas gene editing system applied to trichoderma reesei
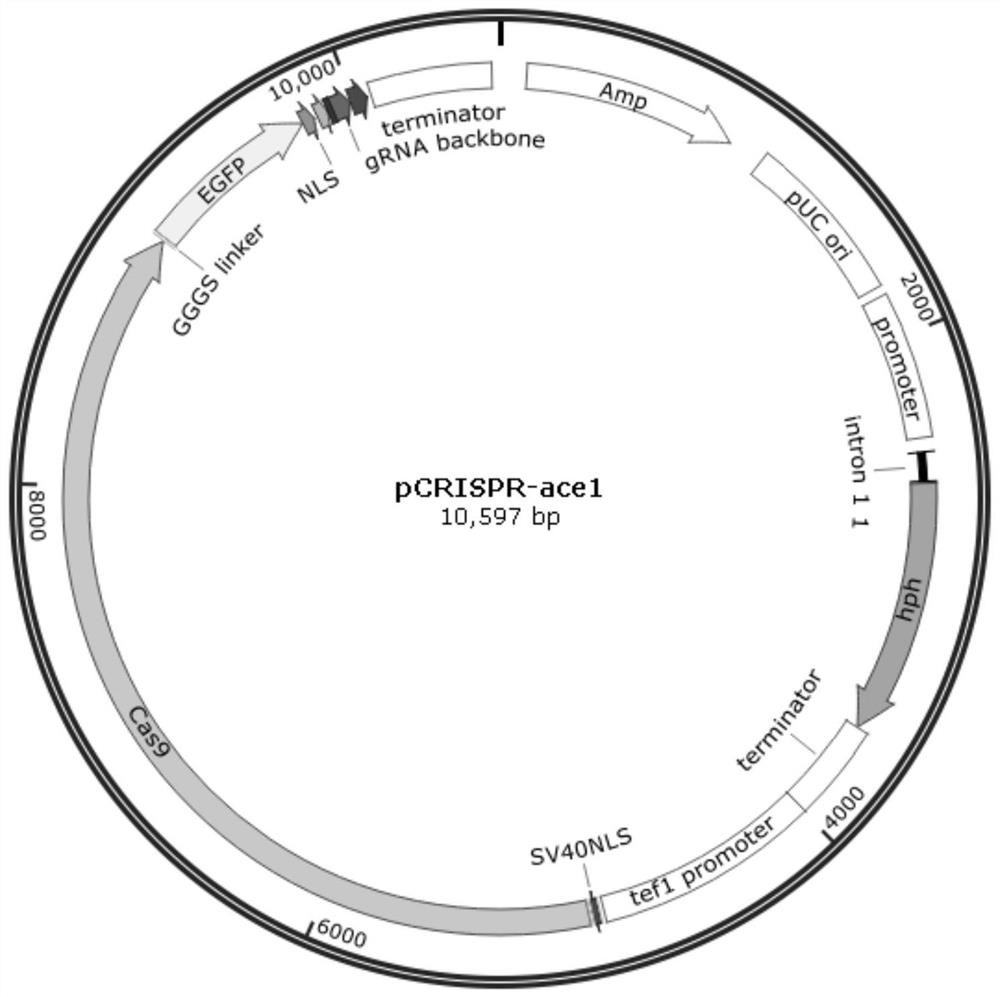
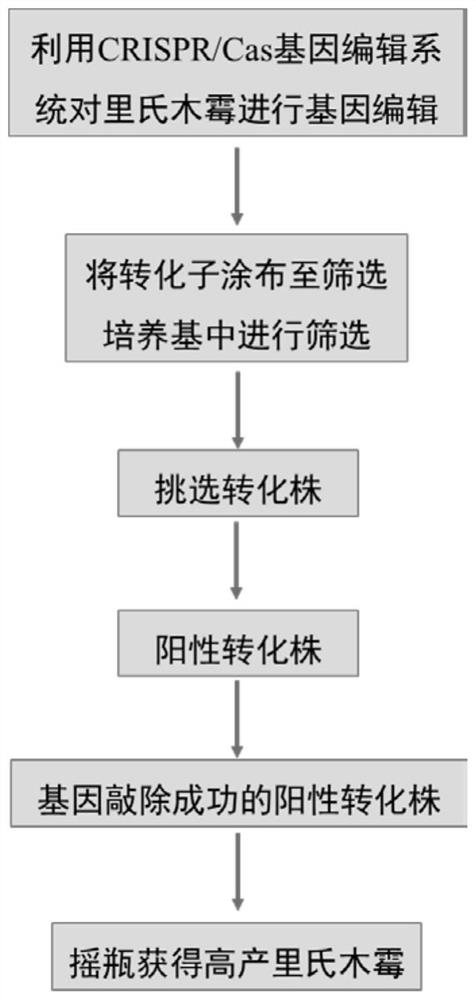
PendingCN113604472AAvoid complexityAvoid stabilityFungiHydrolasesTrichoderma reeseiTranscription start
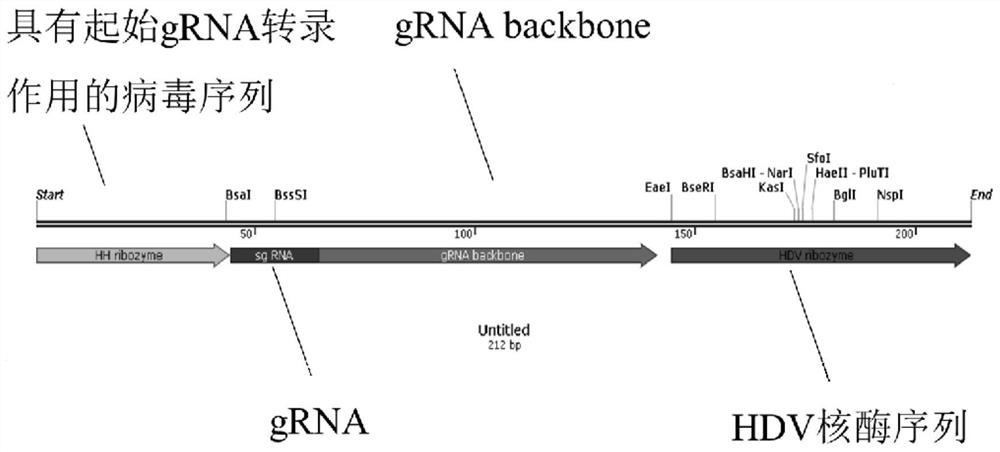
The invention provides a gRNA expression cassette applied to a CRISPR / Cas gene editing system. The gRNA expression cassette has the following structure from 5'-3' that A-B-C-D, A is a virus sequence with a gRNA transcription starting effect, B is gRNA, C is gRNA backbone, D is a hepatitis virus ribozyme (HDV) sequence, and the gRNA expression cassette can be recognized and transcribed by an RNA polymerase II promoter in trichoderma reesei. According to the gRNA expression cassette applied to the CRISPR / Cas gene editing system, simultaneous in-vivo transcription of Cas9 and gRNA is realized in Trichoderma reesei for the first time, and the defects of complexity, instability and the like of transformation after in-vitro transcription of gRNA are overcome.
Owner:INST OF BOTANY CHINESE ACAD OF SCI +1
Construction method of attenuated vaccine against classical swine fever virus with molecular markers
ActiveCN105886531BEfficient and Stable RescueGood genetic stabilitySsRNA viruses positive-sensePeptidesAnti virusBovine Viral Diarrhea Viruses
The invention relates to the field of biotechnology and aims to provide a method for constructing a molecularly marked CSFV (classical swine fever virus) attenuated vaccine. The method comprises following steps: a recombinant plasmid pA-F123 containing genome 5' half-length of a CSFV vaccine strain C and a recombinant plasmid pE-B-Erns containing Erns genes of a BVDV (bovine viral diarrhea virus) strain VEDEVAC are taken as templates, 20bp of homologous fragments are designed at two ends, and by means of a one-step orient cloning kit, a recombinant plasmid pA-B-Erns-F123 is obtained; an F456 fragment containing genome 3' half-length of the CSFV vaccine strain C is cut off from a recombinant plasmid pB-F456 through BamH I and Sal I and cloned into pA-B-Erns-F123 under the action of the same enzyme, and a recombinant plasmid pA-B-Erns-FL is obtained. By virtue of establishment of a reverse genetic manipulation technology, a new way is developed for research of CSFV gene functions and novel vaccines. An exogenous tag is inserted into a viral genome with the reverse genetic manipulation technology for research of virus replication, virus encoded protein functions and anti-virus drug screening, and an RNA (ribonucleic acid) polymerase II system can efficiently and stably save CSFV infectious cDNA (complementary deoxyribonucleic acid ) cloning.
Owner:ZHEJIANG UNIV
Nucleic acid sequence segment for enhancing protein expression
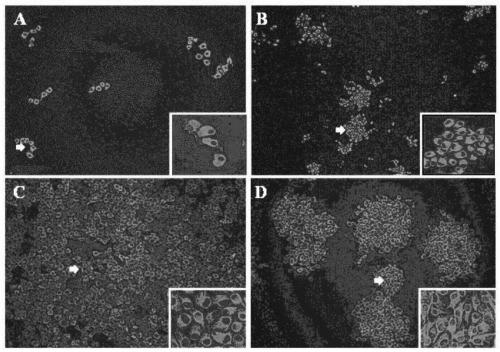
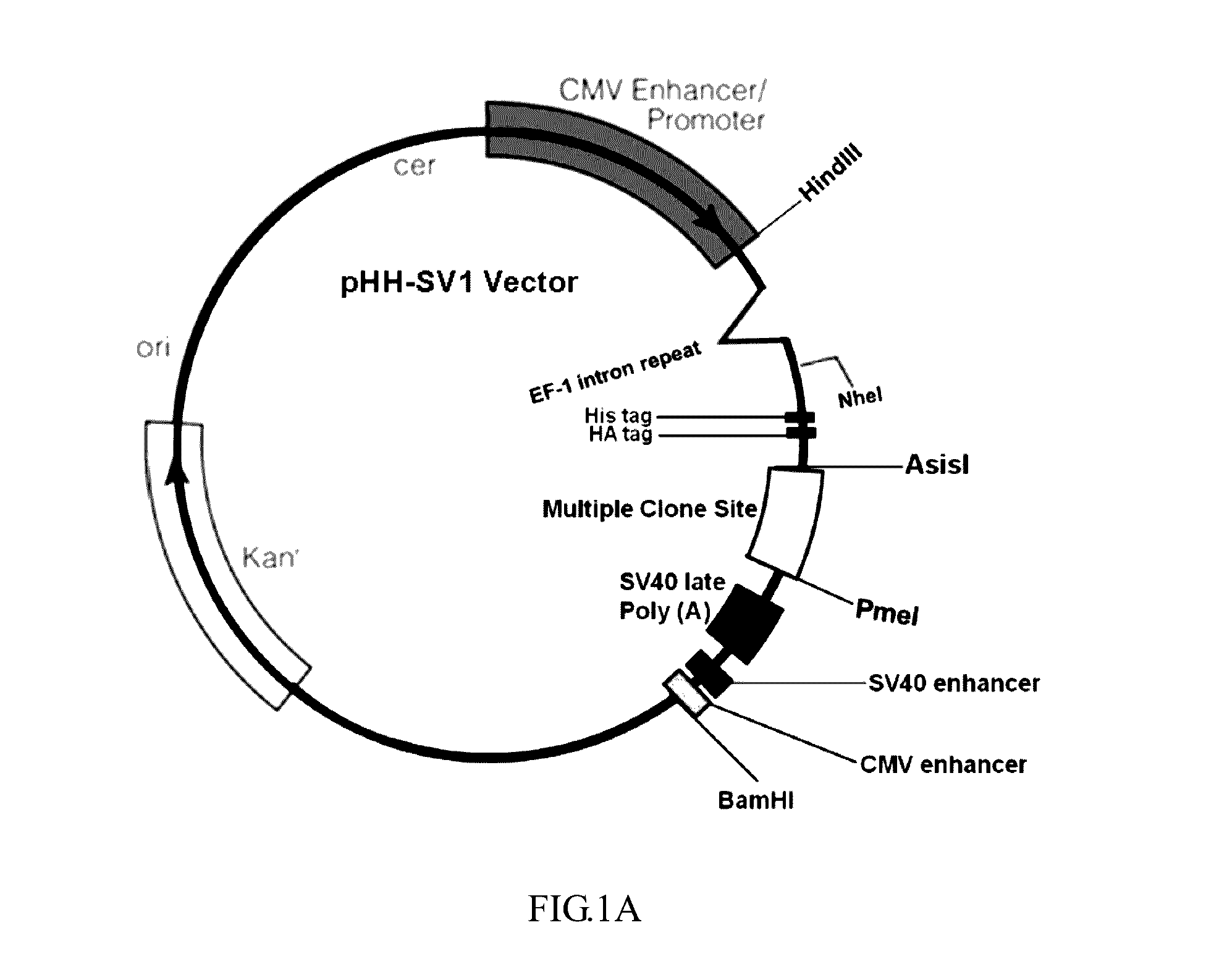
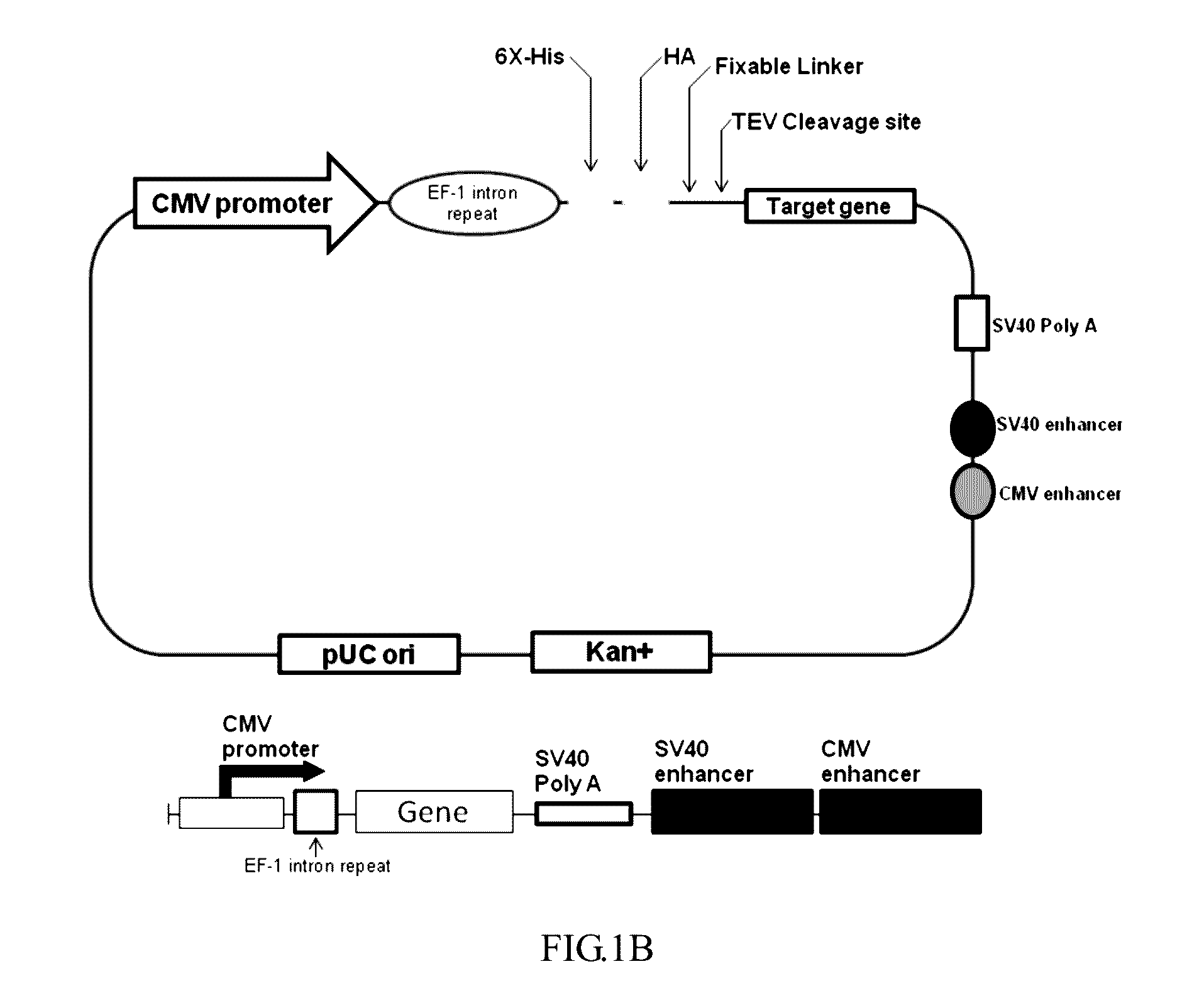
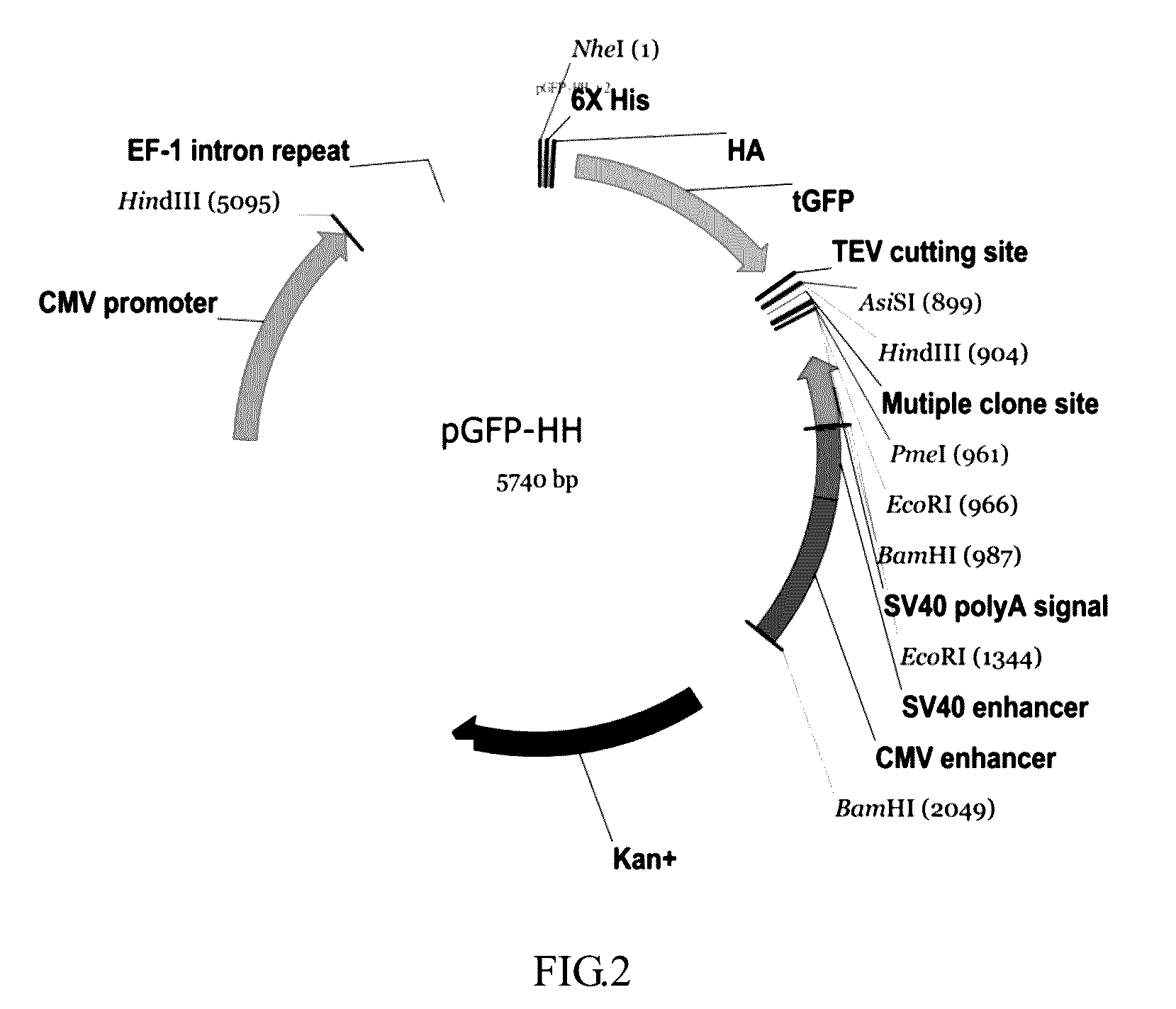
ActiveUS9550983B2Shorten the lengthHigh expressionAntibody mimetics/scaffoldsBiological material analysisProtein insertionRNA polymerase II
An isolated or a purified nucleic acid sequence for enhancing expression levels of a protein of interest, in 5′ to 3′ direction, comprises: a cytomegalovirus (CMV) promoter; an eEF-1 intron repeat (eEF-1 IR); and a regular sequence, which comprises: at least one tag element, a fixable linker sequence, wherein the fixable sequence is TEV sequence; and a multiple cloning site (MCS). By means of the array of the specific promoter and eEF-1 IR, the expression and purity of the recombinant protein could be enhanced; wherein eEF-1 IR can reduce the length of vector and assist RNA polymerase II transcription. Besides, TEV sequence of the fixable linker sequence of the regular sequence can remove a tag on recombinant protein; a specific target can be inserted into the multiple cloning site.
Owner:CHINA MEDICAL UNIVERSITY(TW)
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com