Sustained release microsphere containing risperidone and risperidone analogues and preparation method thereof
A technology of sustained-release microspheres and microspheres, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, and drug combinations, etc. Increased drug release and reduced porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
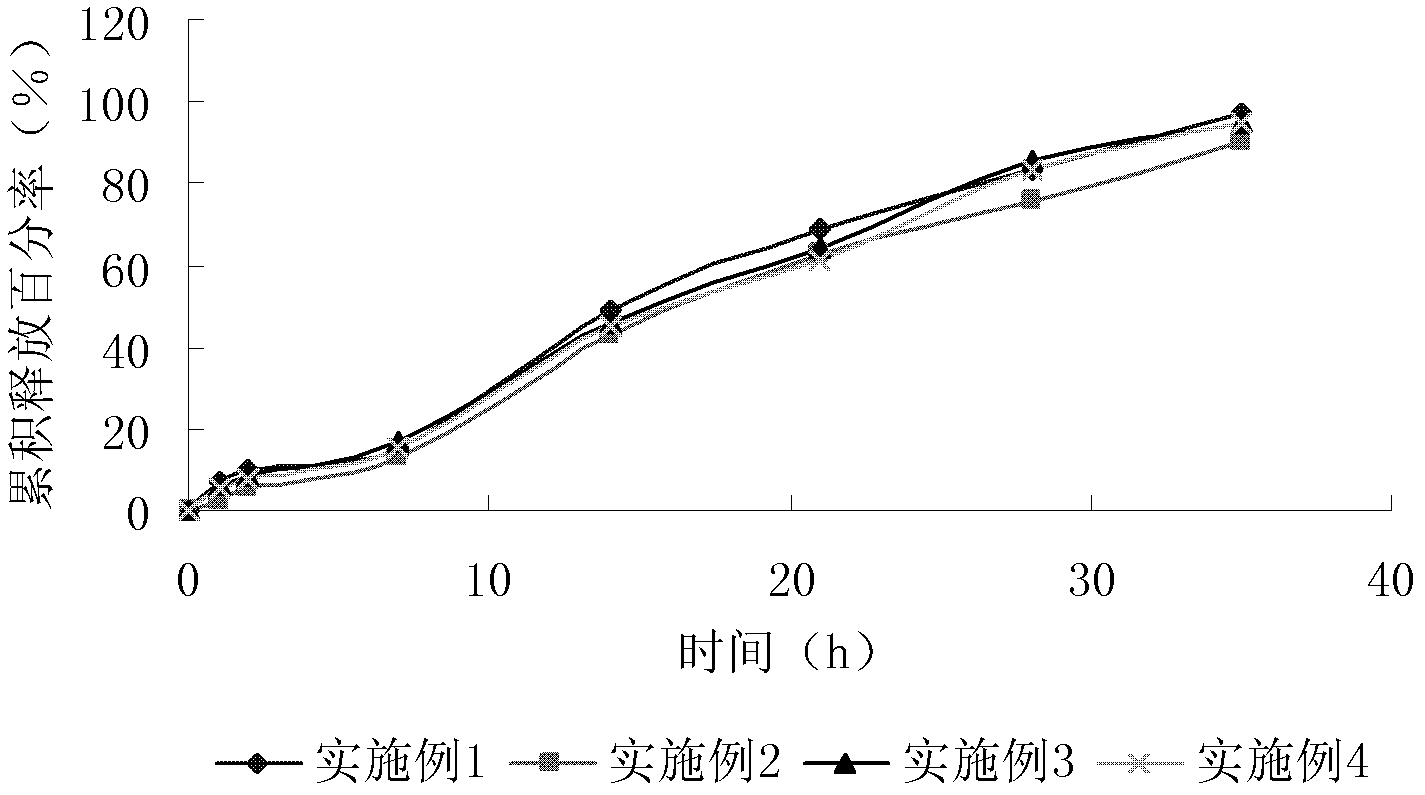
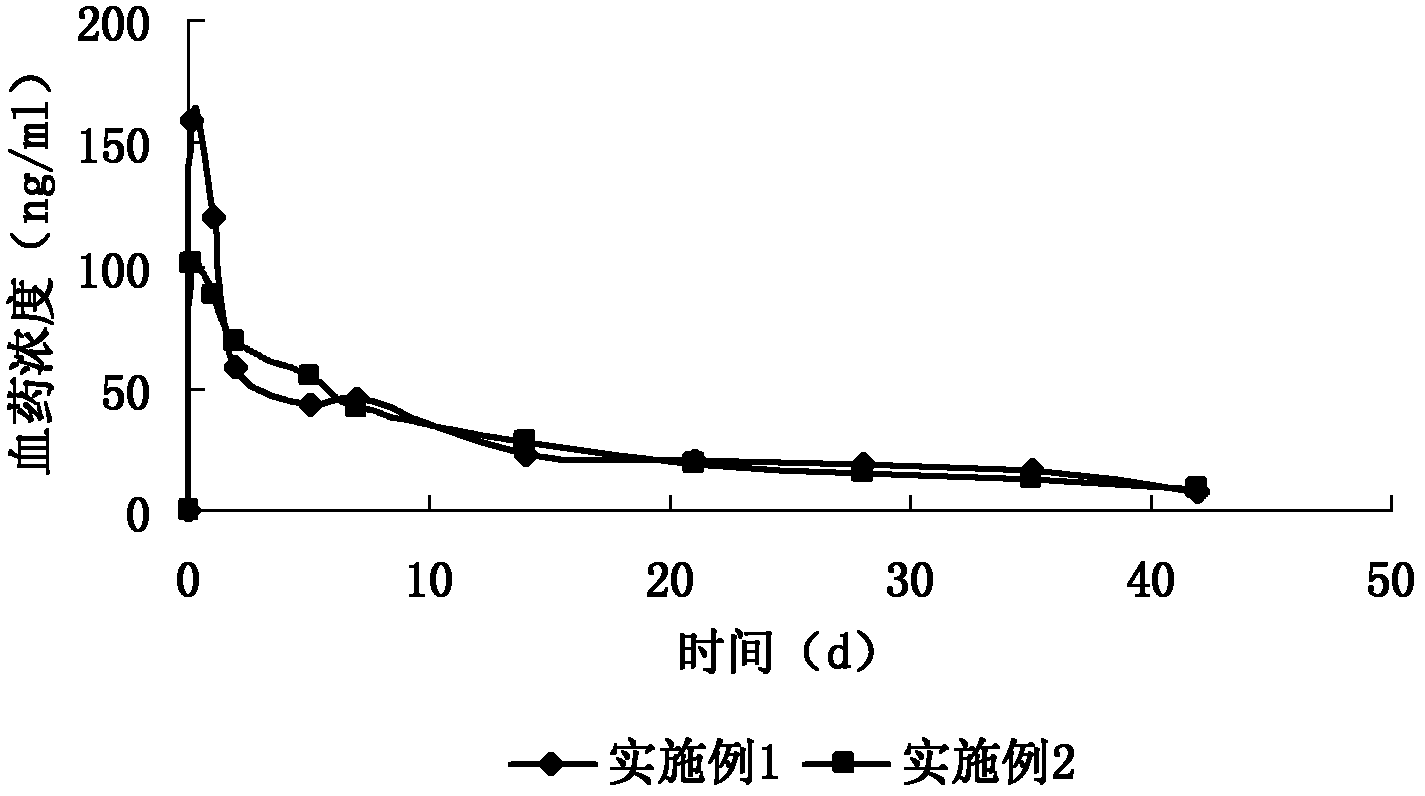
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of 9-hydroxyrisperidone sustained-release microspheres
[0033]28% of the total weight of the microspheres was 9-hydroxyrisperidone (commercially available), 70% of the total weight of the microspheres was PLGA (75:25 ratio of lactic acid and glycolic acid, molecular weight (MW) 100,000), Dibutyl phthalate containing 0.5% of the total weight of the microspheres was dissolved in a mixed solution of dichloromethane and methanol (the volume ratio of dichloromethane and methanol was 8:2), and the solution was added to a mechanically stirred solution at 2500 rpm. In 500 ml of 4% polyvinyl alcohol (PVA) aqueous solution, after 5 minutes, dilute the PVA solution by 1 times, continue stirring for more than 4 hours, then wash, centrifuge and dry to obtain microspheres with an average particle size of 80 μm.
Embodiment 2
[0034] Example 2: Preparation of 9-hydroxyrisperidone sustained-release microspheres
[0035] 28% of the total weight of the microspheres was 9-hydroxyrisperidone (commercially available), 70% of the total weight of the microspheres was PLGA (75:25 ratio of lactic acid and glycolic acid, molecular weight (MW) 100,000), The dibutyl phthalate of 1.9% of the total weight of the microspheres and the tert-butyl-p-hydroxyanisole of 0.1% of the total weight of the microspheres are dissolved in the mixed solution of dichloromethane and methanol (the volume ratio of dichloromethane and methanol is 8:2), add this solution to 500 ml of 3% polyvinyl alcohol (PVA) aqueous solution mechanically stirred at 2500 rpm, dilute the PVA solution 1 times after 5 minutes, continue stirring for more than 4 hours, then wash, centrifuge, and dry. Then take an appropriate amount of the above-mentioned microspheres, put them in a dichloromethane solution containing 300mg / ml PLGA, leave them for 30s, take...
Embodiment 3
[0036] Example 3: Preparation of risperidone sustained-release microspheres
[0037] 38% of the total weight of the microspheres was risperidone (commercially available), 60% of the total weight of the microspheres was PLGA (75:25 ratio of lactic acid and glycolic acid, molecular weight (MW) 150,000), and 60% of the total weight of the microspheres. 1% by weight of dibutyl phthalate and 1% of tert-butyl-p-hydroxyanisole in the total weight of the microspheres are dissolved in a mixed solution of dichloromethane and methanol (the volume ratio of dichloromethane and methanol is 8:2 ), add this solution to 500ml of 3% polyvinyl alcohol (PVA) aqueous solution mechanically stirred at 2500rpm, dilute the PVA solution 1 times with water after 5 minutes, continue stirring for more than 4 hours, then wash, centrifuge, and dry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com