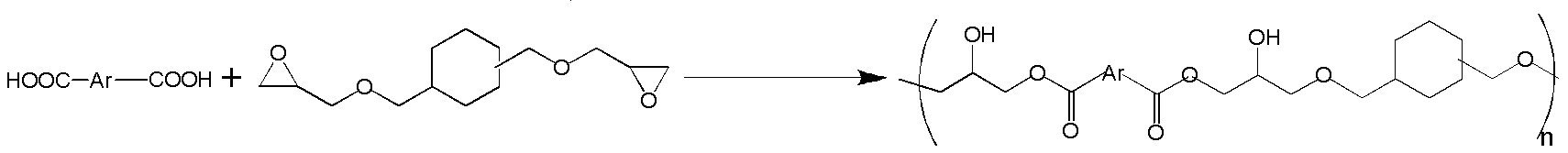Coating compositions
A composition and polymer technology, applied in polyester coatings, epoxy resin coatings, coatings, etc., to achieve high flexibility, good retort resistance, and improved flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0091] The following examples and comparative examples further illustrate the present invention in detail but do not limit the scope of the present invention.
[0092] Various terms and designations are used in the following examples including, for example, those of:
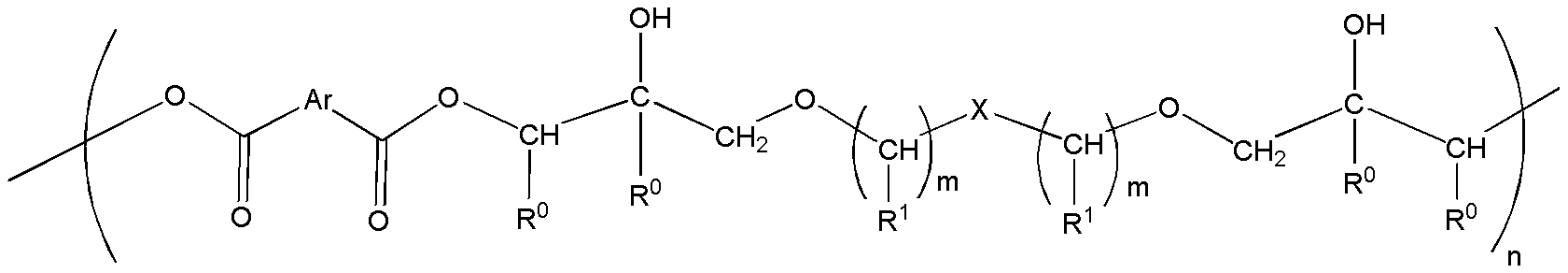
[0093] UNOXOL TM The diols are cis-1,3-cyclohexanedimethanol, cis-1,4-cyclohexanedimethanol, trans-1,3-cyclohexanedimethanol and trans-1,4-cyclohexanedimethanol Mixture of hexanedimethanols available from The Dow Chemical Company. Preparation and purification of diglycidyl ether of cis-1,4-cyclohexanedimethanol, high purity of diglycidyl ether of trans-1,4-cyclohexanedimethanol according to WO2009 / 142901 (measured by gas chromatography >99.0 area %) product mixture and diglycidyl ether of cis-1,3-cyclohexanedimethanol, diglycidyl ether of trans-1,3-cyclohexanedimethanol, cis-1, Diglycidyl ether of 4-cyclohexanedimethanol, diglycidyl ether of trans-1,4-cyclohexanedimethanol (UNOXOL TM Diol DGE) reaction produ...
Embodiment 3
[0125] Example 3 - Curable Compositions and Coatings Prepared from the Polyepoxyester Resins of Synthesis Example 1
[0126] 10.000 g of polyepoxy ester resin from Example 1, 1.111 g of phenolic crosslinker (Methylon 75108), 0.016 g of catalyst (85% phosphoric acid), 0.013 g (BYK-310) additive, 26.666 g of monobutyl A mixture of glycol ether and 6.667 g cyclohexanone was stirred for 16 hours to form a clear solution. The clear solution was filtered through a 1-micron syringe filter, then coated onto tin-free steel (TFS) panels with a #20 coating bar. The resulting coated panels were dried and cured in an oven at 205°C for 15 minutes. The thickness of the cured coating was 5.4 microns.
Embodiment 4
[0127] Example 4 - Curable Compositions and Coatings Prepared from the Polyepoxyester Resins of Synthesis Example 2
[0128] 10.000 g of polyepoxy ester resin from Example 2, 1.111 g of phenolic crosslinker (Methylon 75108), 0.016 g of catalyst (85% phosphoric acid), 0.013 g (BYK-310) additive, 26.666 g of monobutyl ethyl A mixture of glycol ether and 6.667 g cyclohexanone was stirred for 16 hours to form a clear solution. The clear solution was filtered through a 1-micron syringe filter, then coated onto tin-free steel (TFS) panels with a #20 coating bar. The resulting coated panels were dried and cured in an oven at 205°C for 15 minutes. The thickness of the cured coating was 4.3 microns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com