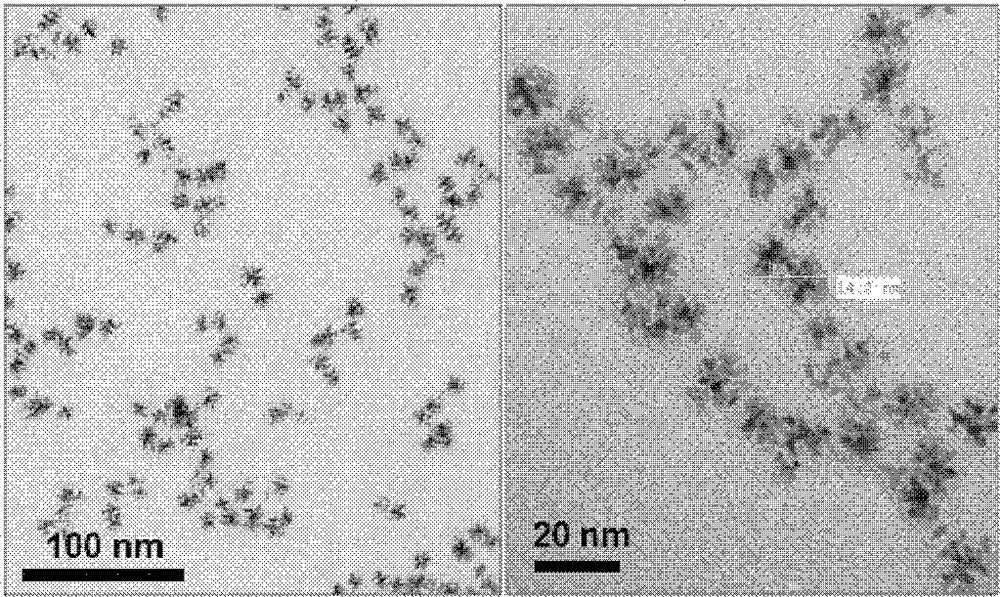
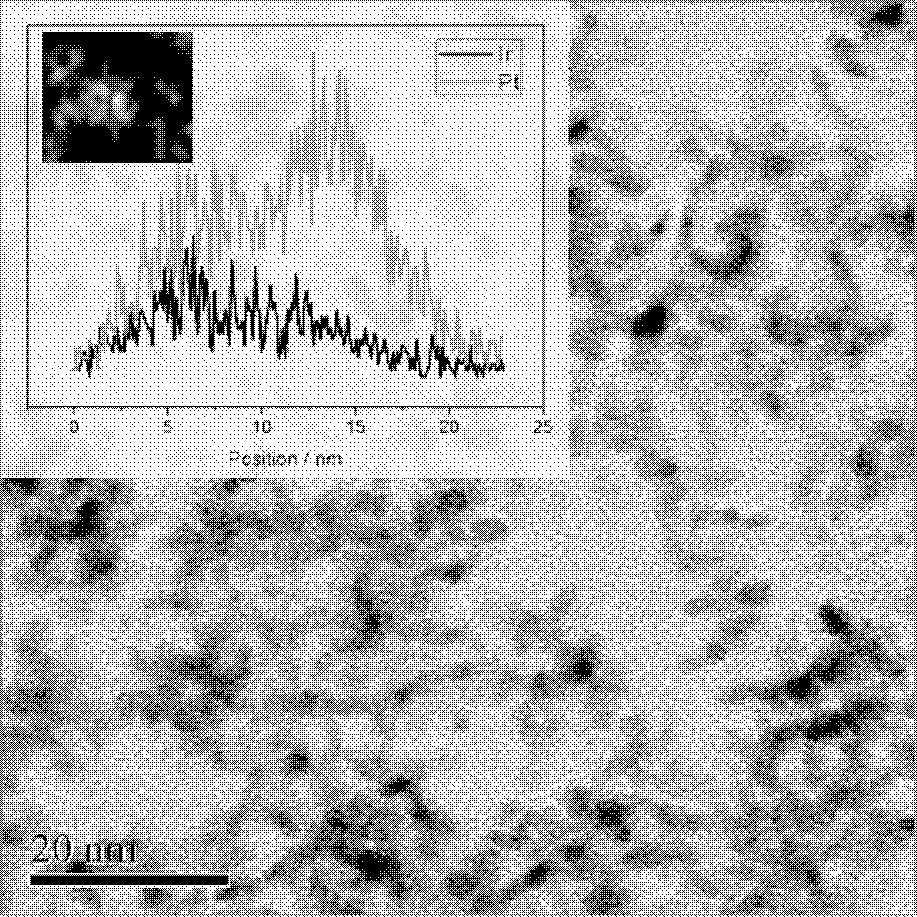
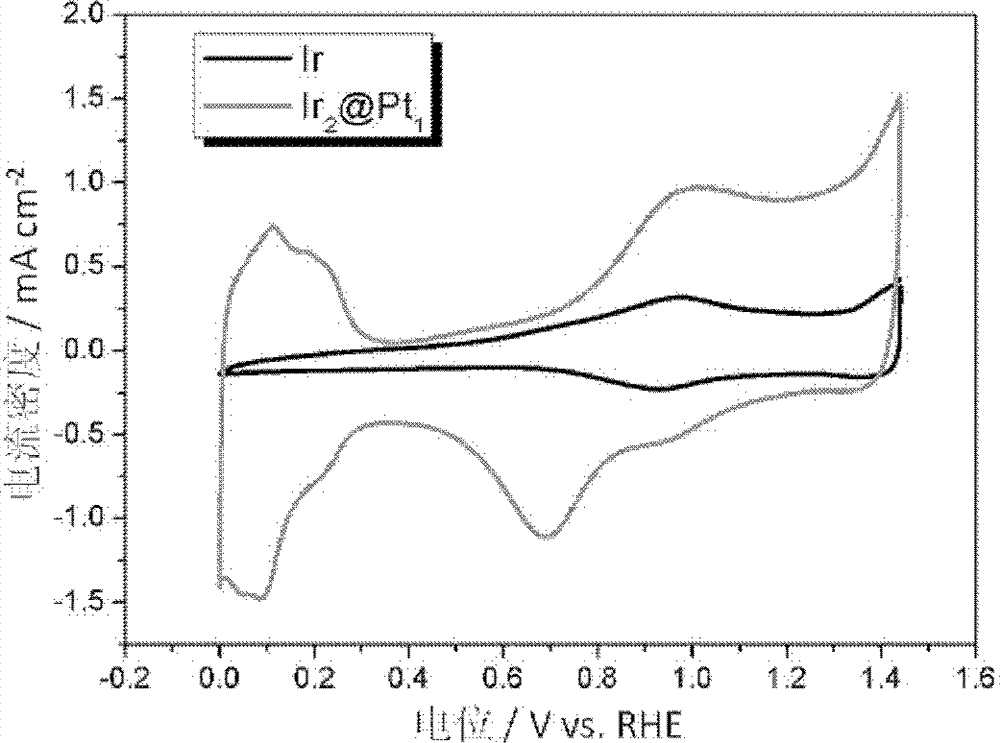
Preparation method of double-effect oxygen electrode catalyst with core-shell structure for fuel cells
A dual-effect oxygen electrode, core-shell structure technology, applied in battery electrodes, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of cumbersome preparation steps and harsh reaction conditions, and achieve Simple and effective preparation method, reduced loading, excellent oxygen reduction activity and oxygen evolution activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Add cetyltrimethylammonium chloride (CTAC) to deionized water and stir to dissolve it completely. The mass fraction of CTAC in the solution is 0.2%.
[0027] 2. Add H to the above solution 2 IrCl 6 , stir evenly so that H 2 IrCl 6 The molar concentration is 1mmol / L.
[0028] 3. Heat the above solution to 60°C, then add NaBH 4 , such that NaBH 4 The ratio of the amount of substance to the Ir element is 30:1, and then react at 60° C. for 5 hours to obtain a sol of Ir.
[0029] 4. Add H to the above Ir sol 2 PtCl 6 , so that the atomic ratio of Pt to Ir is 1:2. Then add ascorbic acid so that the ratio of ascorbic acid to Pt is 30:1, and stir at 60° C. for 6 h.
[0030] 5. Centrifuge after the reaction, wash 3 times with ethanol and water respectively to obtain Ir 2 Pt 1 nanocatalyst.
Embodiment 2
[0032] 1. Add cetyltrimethylammonium chloride (CTAC) to deionized water and stir to dissolve it completely. The mass fraction of CTAC in the solution is 0.2%.
[0033] 2. Add H to the above solution 2 IrCl 6 , stir evenly so that H 2 IrCl 6 The molar concentration is 1mmol / L.
[0034] 3. Heat the above solution to 60°C, then add NaBH 4 , such that NaBH 4 The ratio of the amount of substance to the Ir element is 30:1, and then react at 60° C. for 5 hours to obtain a sol of Ir.
[0035] 4. Add H to the above Ir sol 2 PtCl 6 , so that the atomic ratio of Pt to Ir is 1:1. Then add ascorbic acid so that the ratio of ascorbic acid to Pt is 30:1, and stir at 60° C. for 6 h.
[0036] 5. Centrifuge after the reaction, wash 3 times with ethanol and water respectively to obtain Ir 1 Pt 1 nanocatalyst.
Embodiment 3
[0038] 1. Add Pluronic F127 in deionized water and stir to dissolve it completely. The mass fraction of F127 in the solution is 0.2%.
[0039] 2. Add H to the above solution 2 IrCl 6 , stir evenly so that H 2 IrCl 6 The molar concentration is 0.5mmol / L.
[0040] 3. Heat the above solution to 60°C, then add NaBH 4 , such that NaBH 4 The ratio of the amount of substance to the Ir element is 30:1, and then react at 60° C. for 5 hours to obtain a sol of Ir.
[0041] 4. Add H to the above Ir sol 2 PtCl 6 , so that the atomic ratio of Pt to Ir is 1:2. Then add ascorbic acid so that the ratio of ascorbic acid to Pt is 30:1, and stir at 60° C. for 6 h.
[0042] 5. Centrifuge after the reaction, wash with ethanol and water three times respectively to obtain the IrPt nano-catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com