Blend of fluorine-containing elastomers
A technology of elastomers and blends, applied in the field of fluoroelastomer blends, to achieve the effects of excellent processability, low hardness and low modulus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
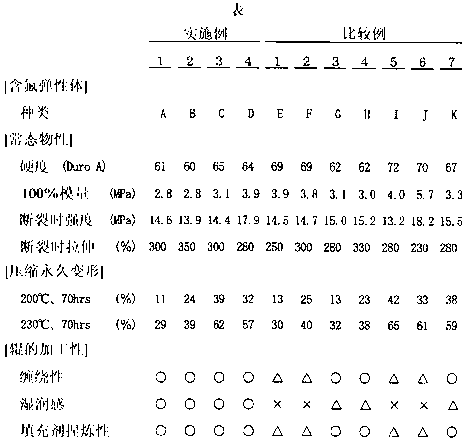
Examples
Embodiment 1
[0056] (1) After substituting and degassing the inside of a stainless steel autoclave with a capacity of 10 L with nitrogen, the following reaction medium was added.
[0057] Surfactant C. 3 f 7 OCF (CF 3 ) CF 2 OCF (CF 3 )COONH 4 8.5g
[0058] Na 2 HPO 4 ?12H 2 O 5.0g
[0059] Ion exchanged water 5000ml
[0060] After the inside of the autoclave was substituted with nitrogen again to degas it, the following reaction raw materials were added.
[0061] Vinylidene fluoride [VdF] 380g (56.0 mol%)
[0062] Hexafluoropropylene [HFP] 700g (44.0mol%)
[0063] Isopropyl alcohol [IPA] 2.0 g (0.065% by weight).
[0064] Next, the temperature in the autoclave was set to 80°C. At this time, it is 32 relative bars (32×10 5 Relative Pa) pressure. Ammonium persulfate [APS] 0.5 g (0.016% by weight) was added thereto as a 0.3% by weight aqueous solution to initiate a polymerization reaction. Keep the pressure at 31-32 relative bar (31-32×10 5 To the state of Pa), the followi...
Embodiment 2
[0080] (1) After substituting and degassing the inside of a stainless steel autoclave with a capacity of 10 L with nitrogen, the following reaction medium was added.
[0081] Surfactant C 3 f 7 OCF (CF 3 ) CF 2 OCF (CF 3 )COONH 4 19.3g
[0082] Na 2 HPO 4 ?12H 2 O 10.0g
[0083] Ion exchanged water 5200ml
[0084] After the inside of the autoclave was substituted with nitrogen again to degas it, the following reaction raw materials were added.
[0085] Vinylidene fluoride [VdF] 101g (34.3 mol%)
[0086] Tetrafluoroethylene [TFE] 49g (10.6 mol%)
[0087] Hexafluoropropylene [HFP] 380g (55.1mol%)
[0088] Isopropyl alcohol [IPA] 2.0 g (0.074% by weight).
[0089] Next, the temperature in the autoclave was set to 80°C. At this time, it is 30 relative bar (30×10 5 Relative Pa) pressure. Ammonium persulfate [APS] 0.8 g (0.030% by weight) was added thereto as a 0.3% by weight aqueous solution to initiate a polymerization reaction. Keep the pressure at 29-30 relati...
Embodiment 3
[0098] (1) After substituting and degassing the inside of a stainless steel autoclave with a capacity of 10 L with nitrogen, the following reaction medium was added.
[0099] Surfactant C 3 f 7 OCF (CF 3 ) CF 2 OCF (CF 3 )COONH 4 19.3g
[0100] Na 2 HPO 4 ?12H 2 O 10.0g
[0101] Ion exchanged water 5200ml
[0102] After the inside of the autoclave was substituted with nitrogen again to degas it, the following reaction raw materials were added.
[0103] Vinylidene fluoride [VdF] 101g (34.3 mol%)
[0104] Tetrafluoroethylene [TFE] 49g (10.6 mol%)
[0105] Hexafluoropropylene [HFP] 380g (55.1mol%)
[0106] 2-Bromo-1,1-difluoroethylene [BDFE] 8.3g
[0107] Octafluoro-1,4-diiodobutane [DIOFB] 3.0 g (0.111% by weight).
[0108] Next, the temperature in the autoclave was set to 80°C. At this time, it is 30 relative bar (30×10 5 Relative Pa) pressure. 1.0 g (0.037% by weight) of ammonium persulfate [APS] was added thereto as a 0.3% by weight aqueous solution to init...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com