Method for preparing p-cresol into p-hydroxy benzaldehyde by catalytic oxidation of metalloporphyrin-metal salt composite catalyst
A technology of p-hydroxybenzaldehyde and composite catalyst, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. Less, lower resource consumption and operating costs, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
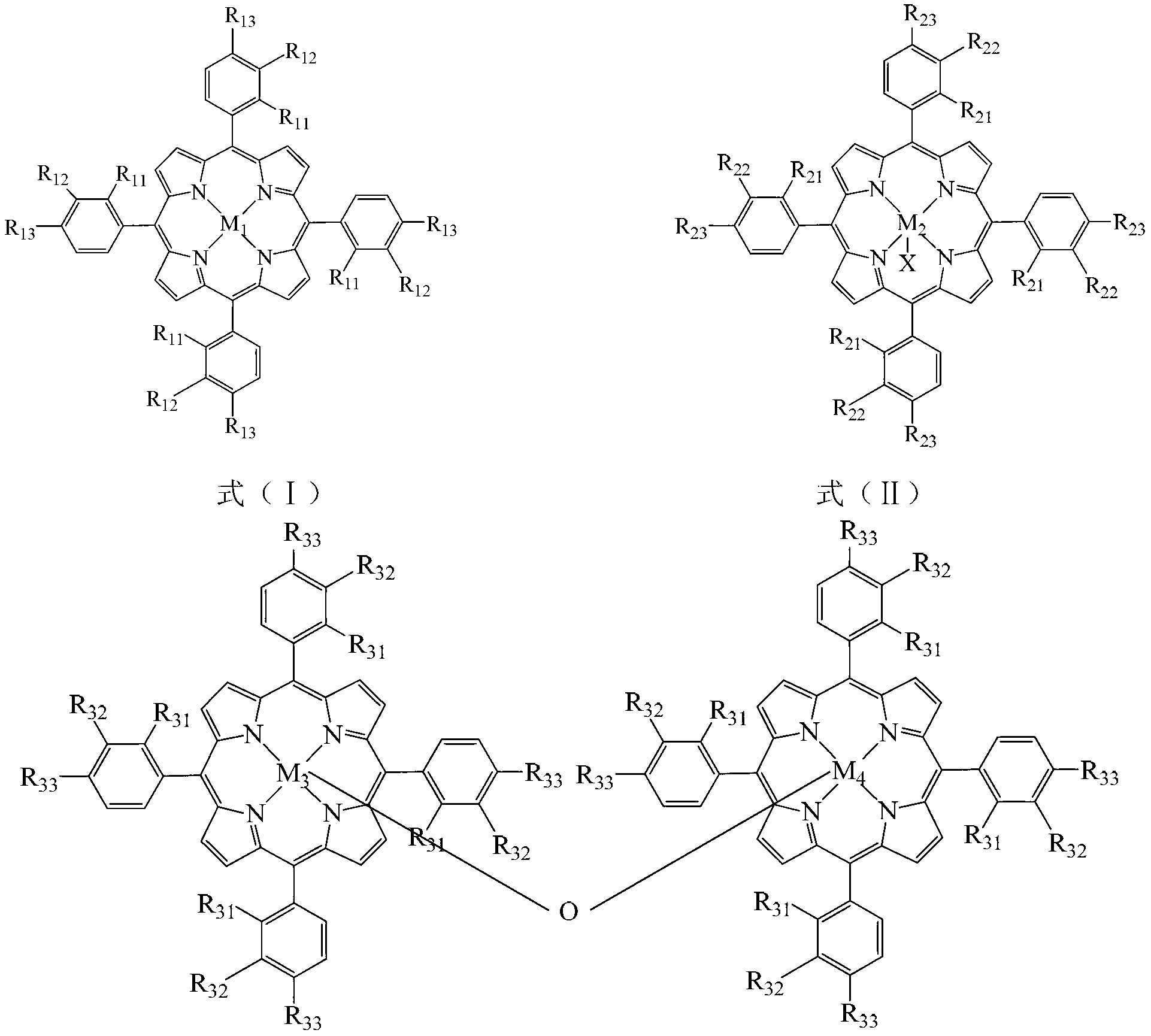
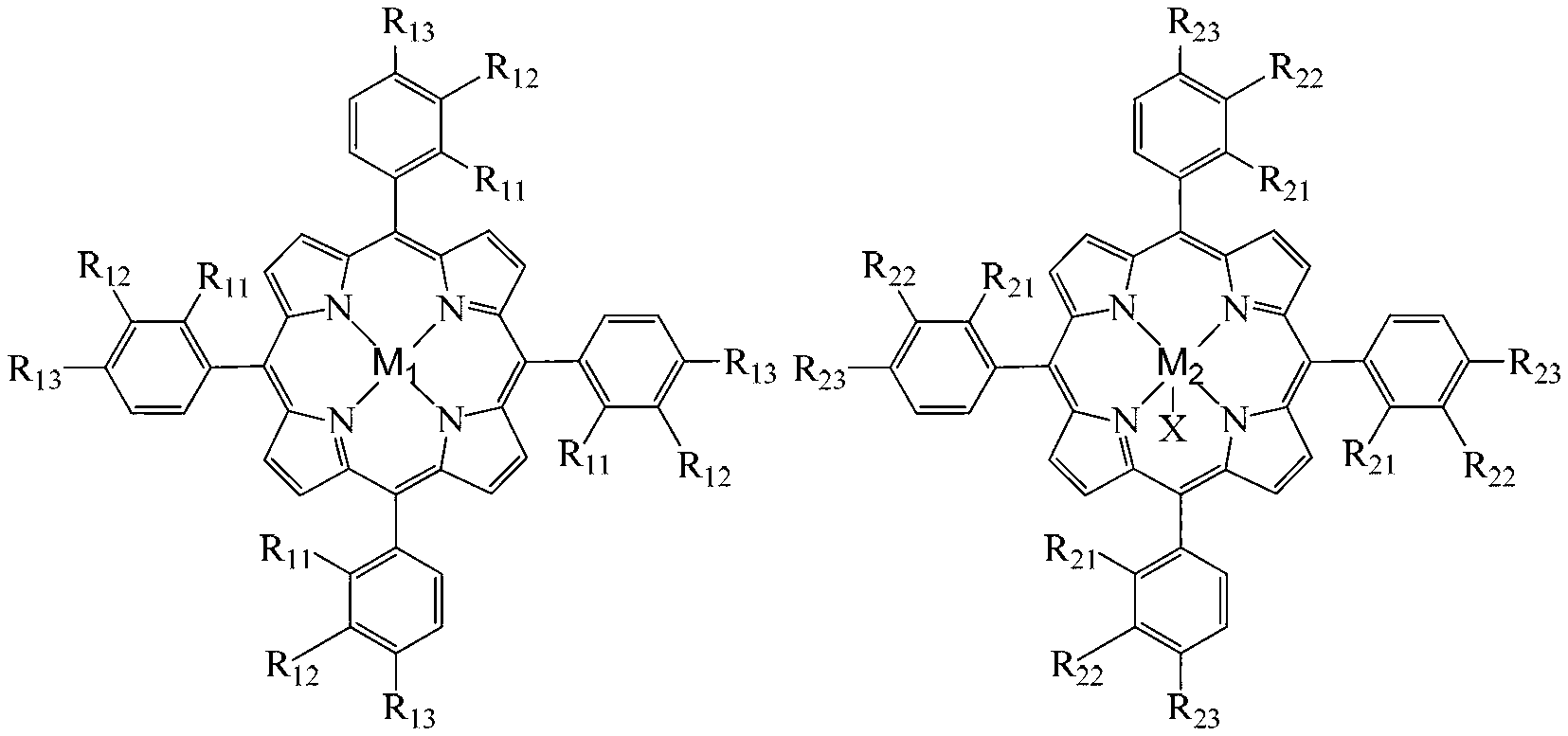
[0022] Take 6.4×10 -3 g chloride tetra-(p-methoxyphenyl) cobalt porphyrin (i.e. R in the general formula (II) 21 =R 22 = H, R 23 =OCH 3 , X=Cl,M 2 =Co), 3.2×10 -2 Add 1 g of ferrous acetate, 12.0 g of p-cresol and 12.0 g of sodium hydroxide into a 100 mL autoclave, add 40 mL of methanol, feed oxygen at a pressure of 0.2 MPa, and react in a water bath at a temperature of 65°C for 10 h. After the reaction was completed, the reaction liquid was detected by high performance liquid chromatography, and the conversion rate of p-cresol was 99.2%, the selectivity of p-hydroxybenzaldehyde was 80.0%, and the yield of p-hydroxybenzaldehyde was 79.4%.
Embodiment 2
[0024] Take 8.6×10 -4 g chloride tetra-(p-nitrophenyl)iron porphyrin (i.e. R in the general formula (II) 21 =R 22 = H, R 23 =NO 2 , X=Cl,M 2 =Fe), 3.8×10 -4 Add 1g zinc chloride, 16.0g p-cresol and 8.9g sodium hydroxide into a 100mL autoclave, add 40mL methanol, feed oxygen at a pressure of 0.6MPa, and react in a water bath at a temperature of 70°C for 5h. After the reaction was completed, the reaction solution was detected by high performance liquid chromatography, and the conversion rate of p-cresol was 83.0%, the selectivity of p-hydroxybenzaldehyde was 67.4%, and the yield of p-hydroxybenzaldehyde was 55.9%.
Embodiment 3
[0026] Take 6.4×10 -3 g chloride tetra-(p-methoxyphenyl)iron porphyrin (i.e. R in the general formula (II) 11 =R 12 = H, R 13 =OCH 3 , X=Cl,M 2 =Fe), 1.8×10 -3 Add g cobalt acetate, 16.0 g p-cresol and 14.5 g sodium hydroxide into a 100 mL autoclave, add 40 mL methanol, feed oxygen at a pressure of 0.2 MPa, and react in a water bath at 65°C for 10 h. After the reaction was completed, the reaction liquid was detected by high performance liquid chromatography, and the conversion rate of p-cresol was 99.9%, the selectivity of p-hydroxybenzaldehyde was 87.1%, and the yield of p-hydroxybenzaldehyde was 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com