Lycorine alkaline compound with neuroprotective effect
A technology of neuroprotection and compounds, applied in the field of medicine, can solve the problems of unclear pathogenic mechanism, treatment, and failure to achieve therapeutic effect of AD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
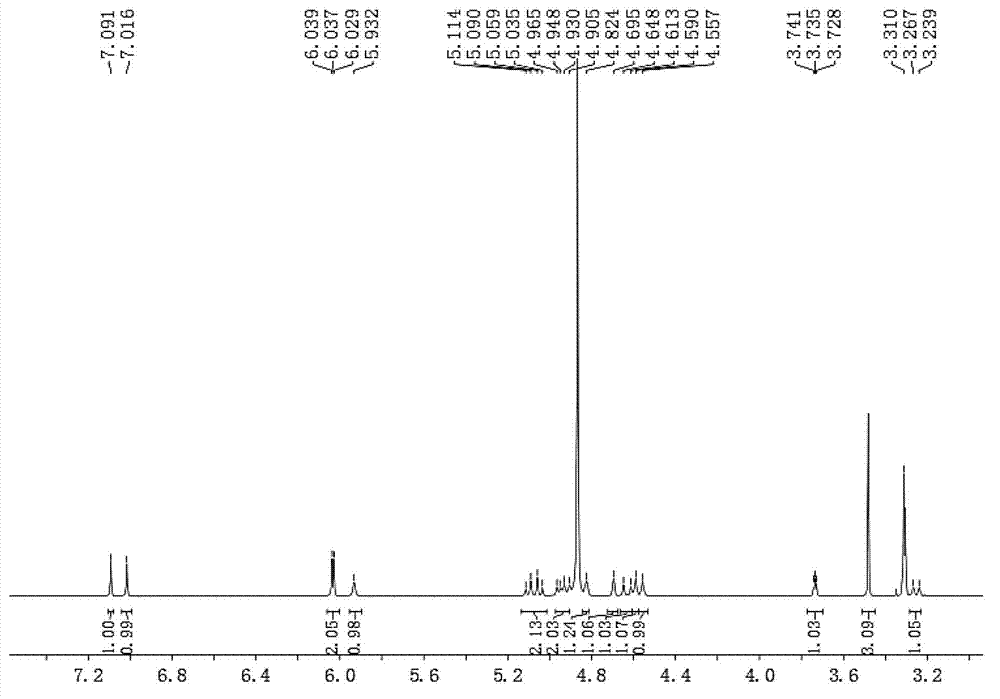
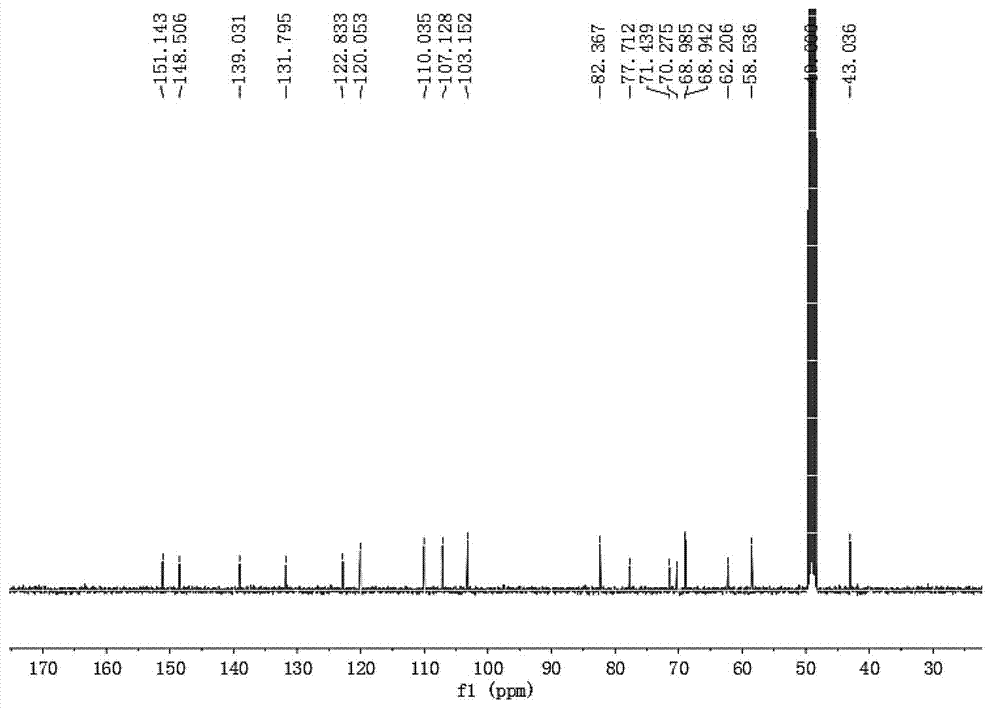
[0031] The preparation method of embodiment 1 hybrid lycorine
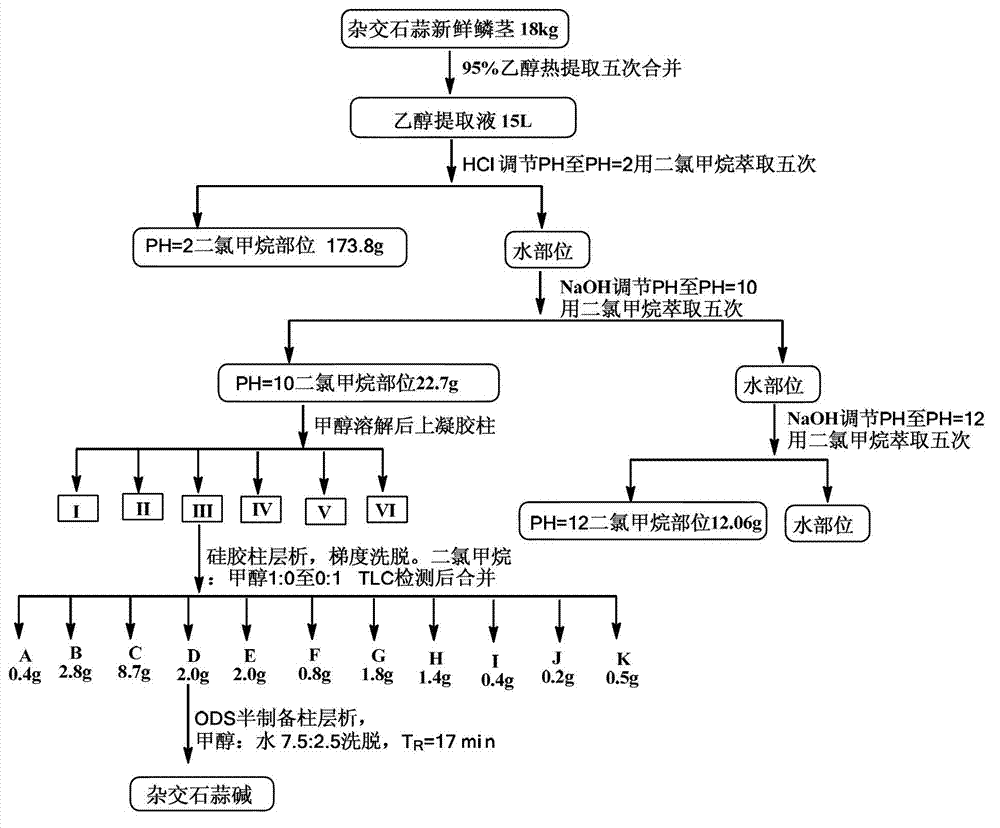
[0032] 18 kg of fresh bulbs of Hybridized Lycoris were chopped, heated with 95% ethanol for 5 times (2 hours each time), the extracts were combined (about 15 L), concentrated under reduced pressure to remove most of the ethanol, and the extract was evenly dispersed in water. Adjust the pH value of the aqueous phase to 2, 10 and 12 with dilute hydrochloric acid and sodium hydroxide solution, and extract with dichloromethane to obtain three dichloromethane sites: 173.8g (when the aqueous phase pH=2), 22.7g (the aqueous phase pH=10) and 12.06g (water phase pH=12).
[0033]The dichloromethane pH 10 extraction fraction (water phase pH=10, 22.7 g) was dissolved in methanol and then subjected to gel chromatography and thin layer chromatography to detect and combine the same fractions to obtain 6 fractions (I-VI). Part III crude silica gel mixed sample, 200g silica gel column chromatography, dichloromethane:methanol 1:0 ...
Embodiment 2
[0034] Example 2 Hybrid lycorine to H 2 o 2 Protective effect on induced neuronal injury
[0035] The results showed that the hybrid lycorine on H 2 o 2 Induced SH-SY5Y nerve cell injury has a protective effect. At 12.5, 25, and 50 μM concentrations, there was a very significant difference between the cell survival rate of the administration group and the model group (P2 o 2 Induced neuronal damage.
[0036] 1. Experimental materials
[0037] 1.1 Test sample
[0038] Hybridized lycorine was dissolved in DMSO (sigma) and configured as a 10000 μM stock solution, and then diluted to the required concentration with DMEM medium.
[0039] 1.2 Cell lines
[0040] SH-SY5Y (human neuroblastoma cells)
[0041] 1.3 Culture medium
[0042] DMEM+10%NBS+double antibody
[0043] 1.4 Other instrument materials
[0044] BioTek-Synergy2 multifunctional microplate reader, cell culture incubator, ultra-clean bench, 96-well culture plate, cell culture flask, etc. 30%H 2 o 2 Purchase...
Embodiment 3
[0052] Example 3 Hybridization of lycorine to CoCl 2 Protective effect on induced neuronal injury
[0053] The results showed that the hybrid lycorine had a positive effect on CoCl 2 Induced SH-SY5Y nerve cell injury has a protective effect. At concentrations of 12.5, 25, 50, and 100 μM, there were extremely significant differences between the cell survival rate of the administration group and the model group (P2 Induced neuronal damage.
[0054] 1. Experimental materials
[0055] 1.1 Test sample
[0056] ditto
[0057] 1.2 Cell lines
[0058] ditto
[0059] 1.3 Culture medium
[0060] ditto
[0061] 1.4 Other instrument materials
[0062] Ditto. CoCl 2 Purchased from sigma, prepared to a concentration of 300μM when used, freshly prepared before each use.
[0063] 2. Experimental method
[0064] MTT method: 3-4×10 4 cells / ml of cell suspension 92 μL, placed at 37 ° C, 5% CO 2 Inside the incubator. After 22 hours of cell attachment, add the sample to be tested, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com