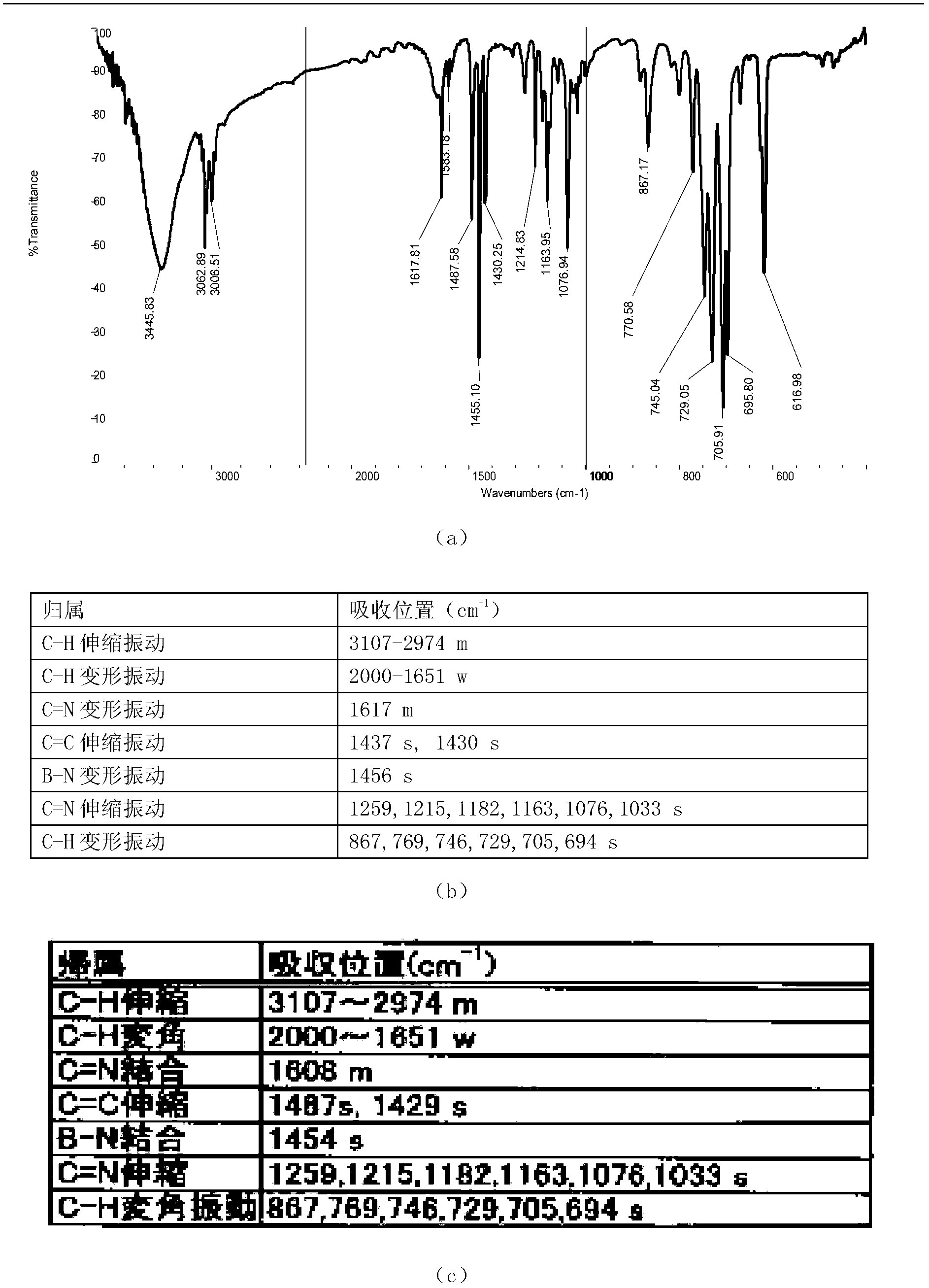
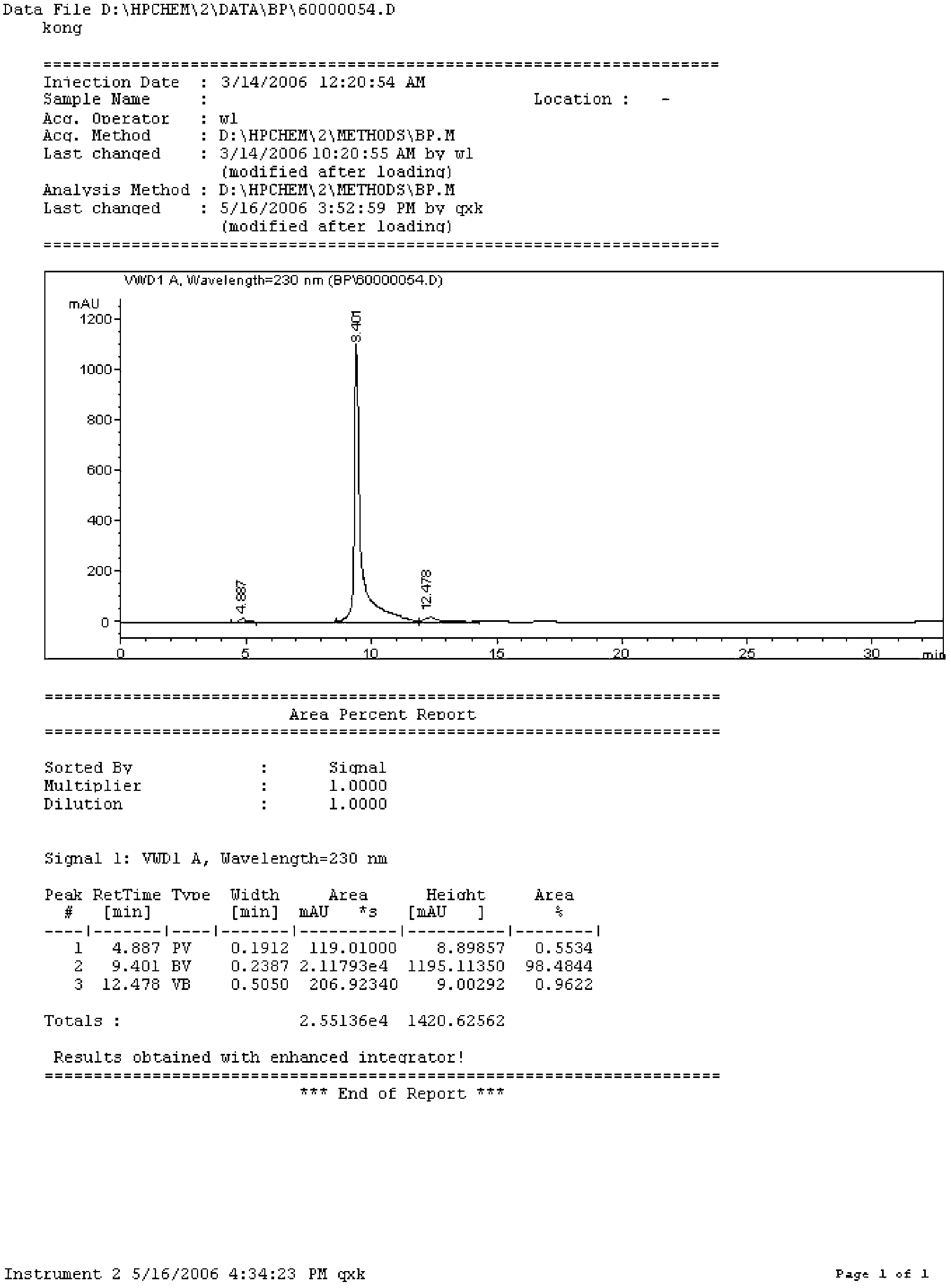
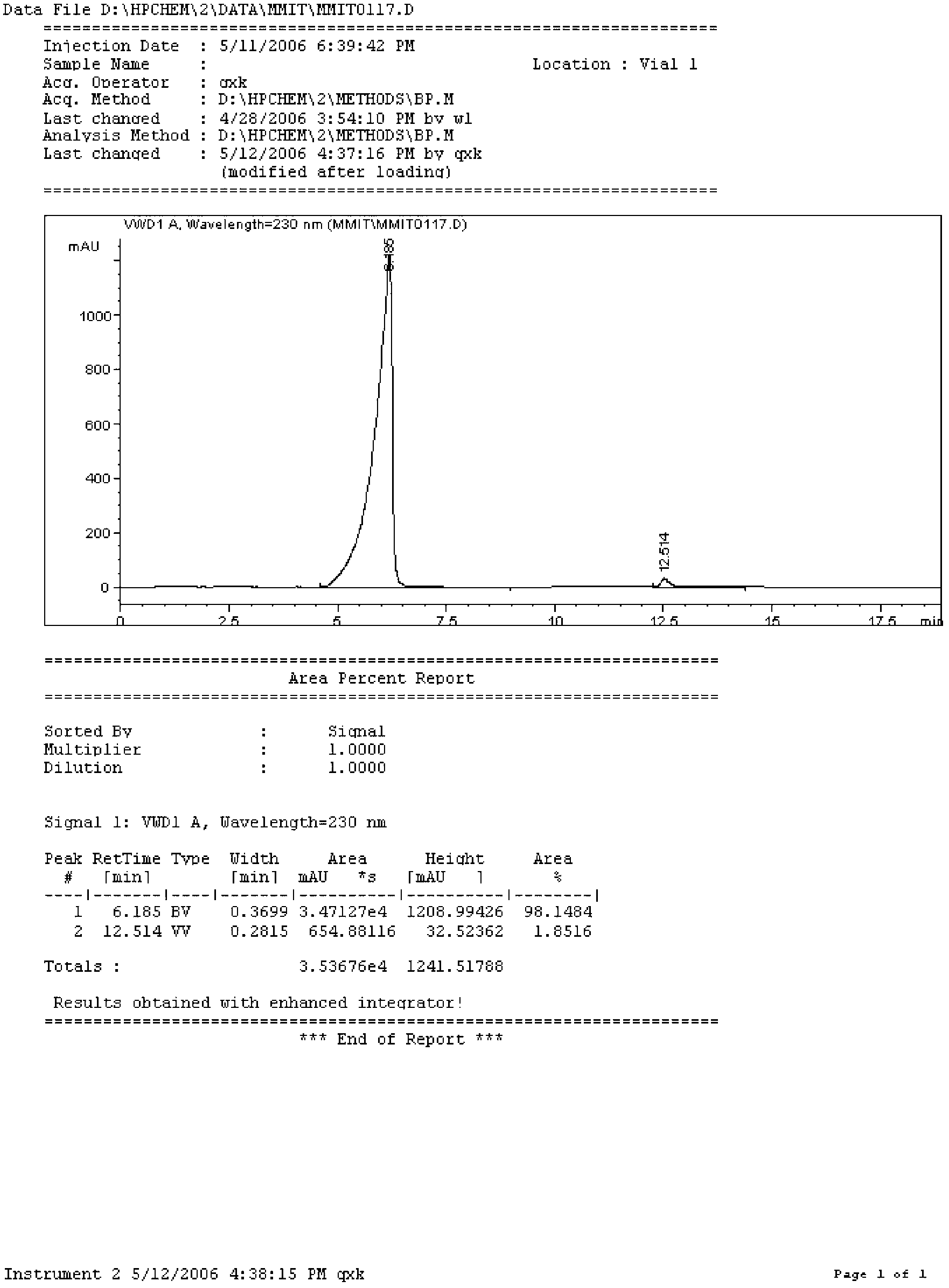
Preparation method of borphenyl pyridine
A technology of triphenylboron pyridine and tetraphenylboron chloride, which is applied in the field of simple synthesis of a new type of organoboron fungicide - triphenylboron pyridine, can solve the problems of limited use of solvents, difficult control of product purity, uneven heating, etc. , to achieve the effect of low cost, high product purity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: a kind of preparation method of triphenylboron pyridine is realized by the following steps:
[0036] The input amount of each substance used in the preparation is based on trimethyl borate / mL=(15.8-19.3) as the standard, the solid is measured by mass, and the liquid is measured by volume.
[0037] (1) Preparation of Phenylmagnesium Chloride (Grignard Reagent) Add 16.0g of magnesium powder and 0.1g of iodine to a 500mL three-necked flask equipped with a reflux condenser (top-mounted tee), dropping funnel and magnetic stirring, and use For argon replacement, first add 2.5mL bromobenzene and 40mL tetrahydrofuran (THF) into the reaction flask, and stir until the reaction is initiated (the color of iodine disappears and a large amount of heat is released). Add 66.0 mL of chlorobenzene and 160.0 mL of tetrahydrofuran into the dropping funnel. After the oil bath was heated to 70°C, the mixed solution of chlorobenzene and tetrahydrofuran was slowly added dropwise...
Embodiment 2
[0051] Embodiment 2: a kind of preparation method of triphenylboron pyridine is realized by following steps:
[0052] The input amount of each substance used in the preparation is based on trimethyl borate / mL=(15.8-19.3) as the standard, the solid is measured by mass, and the liquid is measured by volume.
[0053] (1) Preparation of Phenylmagnesium Chloride (Grignard Reagent) Add 16.0g of magnesium powder and 0.1g of iodine to a 500mL three-necked flask equipped with a reflux condenser (top-mounted tee), dropping funnel and magnetic stirring, and use For argon replacement, first add 2.5mL bromobenzene and 40mL tetrahydrofuran (THF) into the reaction flask, and stir until the reaction is initiated (the color of iodine disappears and a large amount of heat is released). Add 66.0 mL of chlorobenzene and 160.0 mL of tetrahydrofuran into the dropping funnel. After the oil bath was heated to 60°C, the mixed solution of chlorobenzene and tetrahydrofuran was slowly added dropwise for...
Embodiment 3
[0067] Embodiment 3: a kind of preparation method of triphenylboron pyridine is realized by following steps:
[0068] The input amount of each substance used in the preparation is based on trimethyl borate / mL=(15.8-19.3) as the standard, the solid is measured by mass, and the liquid is measured by volume.
[0069] (1) Preparation of Phenylmagnesium Chloride (Grignard Reagent) Add 16.0g of magnesium powder and 0.1g of iodine to a 500mL three-necked flask equipped with a reflux condenser (top-mounted tee), dropping funnel and magnetic stirring, and use For argon replacement, first add 2.5mL bromobenzene and 40mL tetrahydrofuran (THF) into the reaction flask, and stir until the reaction is initiated (the color of iodine disappears and a large amount of heat is released). Add 66.0 mL of chlorobenzene and 160.0 mL of tetrahydrofuran into the dropping funnel. After the oil bath was heated to 40°C, a mixed solution of chlorobenzene and tetrahydrofuran was slowly added dropwise for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com