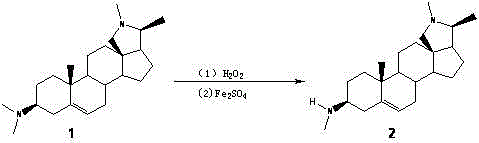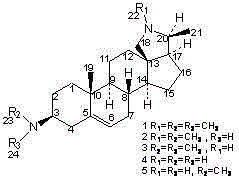Holarrhine alkaloid derivative and application of holarrhine alkaloid derivative
A technology of alkaloid derivatives and antidiarrheal wood, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problems of large dosage and weak effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: conespinine (compound 1 ) preparation
[0031] Put 2 kg of crushed antidiarrheal wood (powder) into a 20L round-bottomed flask, then reflux extract with 13L 90% ethanol for 3 hours, filter, filter the filter residue with 12L 90% ethanol for reflux extraction for 2 hours, filter, combine the two filtrates and concentrate.
[0032] Dissolve the resulting solid in water, then transfer it to a 5L beaker, adjust the pH to 2 with 4M HCl, filter after standing overnight, adjust the pH to 11 with 4M NaOH, then extract with an equal volume of chloroform, and distill under reduced pressure to obtain 200g of total alkaloids of B.
[0033] The total alkaloids of Zhixie wood were heated and dissolved with redistilled ethanol, and then separated and purified with macroporous resin.
[0034] Gradient elution was performed with 20%, 30%, 40%, 50%, 60%, 70%, and 80% ethanol in water.
[0035] will contain conespinine (compound 1 ) components were combined, and then se...
Embodiment 2
[0038] Example 2: N-3 demethylated derivatives of antidiarrheal wood alkaloids (compound 2 ) preparation
[0039] a step reaction:
[0040] 2 g (6 mmol) of dried antidiarrheal alkaloids (compound 1 ) into a 50mL round bottom flask, dissolved in 20mL of anhydrous methanol, then added dropwise 5mL of 30% hydrogen peroxide, and reacted overnight at room temperature.
[0041] Add 10 mL of distilled water to the reaction solution, stir evenly, extract 3 times with chloroform, extract the solution after drying with sodium sulfate, filter and concentrate, and purify by silica gel column chromatography to obtain the compound 1 3-position N-oxide.
[0042] Step b reaction:
[0043] the above compound 1Add the 3-position N oxide into a 50mL round bottom flask, dissolve it with 20mL of anhydrous methanol, add FeSO 4 ·7H 2 0 3.3g (12mmol), react overnight under nitrogen protection at room temperature.
[0044] Then add 10 mL of saturated NaHCO to the reaction soluti...
Embodiment 3
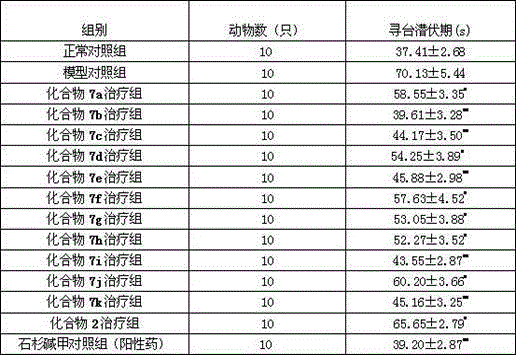
[0048] Example 3: Preparation of 2-bromoethylphenol ethers 6a-6k.
[0049] Add 0.01mol of phenol, 0.5g (0.012mol) of NaOH, 0.05g of TBAB, and 0.05g of KI into a three-necked flask, and add 20mL of distilled water.
[0050] Heat to completely dissolve the ingredients.
[0051] When the temperature reaches 90°C, start to drop 1.3mL (0.015mol) of 1,2-dibromoethane, control the reaction at about 100°C, and react for 5h.
[0052] After the reaction was completed, it was cooled, and the water layer was removed. The organic layer was washed twice with 2% NaOH aqueous solution, once with saturated sodium chloride, dried over sodium sulfate, filtered, and concentrated.
[0053] Silica gel column chromatography purification.
[0054] 2-bromoethylphenol ether 6a-6k (yield 80% - 90%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com