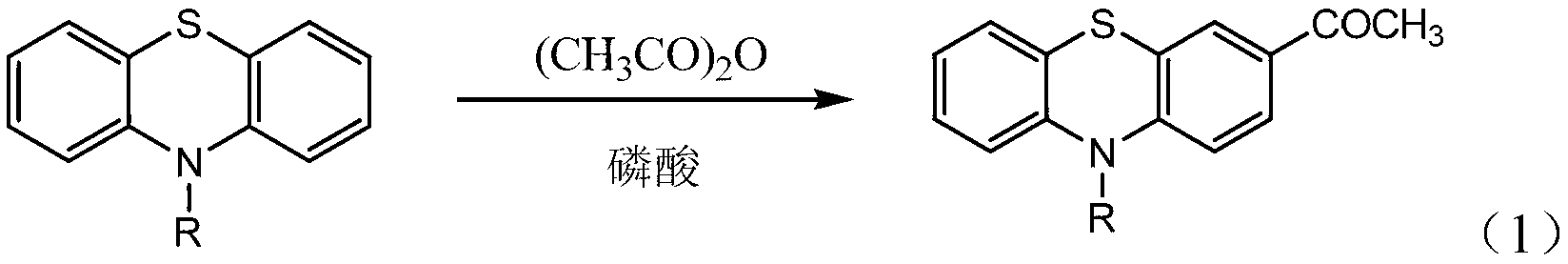
Method for preparing 3-acetyl-10-alkyl phenothiazine
A technology of alkyl phenothiazine and acetyl, which is applied in the field of preparing 3-acetyl-10-alkyl phenothiazine, can solve the problems of high raw material cost, long reaction time, cumbersome process, etc., and achieve short reaction time, The effect of simple reaction process and simple post-processing operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the first step, add 1 mol of 10-methylphenothiazine, 7.1 mol of acetic anhydride and 1.0 mol of phosphoric acid into a dry three-necked flask, and react at 20°C for 2 hours to obtain a reaction solution;
[0023] The second step is to cool the reaction liquid to room temperature, pour it into 8mL water, filter after the solid is precipitated, wash the filter cake with water, and dry it with a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:5. Recrystallize the filter cake to obtain 3-acetyl-10-methylphenothiazine with a yield of 85.5%.
[0024] IR (υ max , KBr, cm -1 ): 3050, 2976, 1725, 1324, 765. 1 HNMR (δppm, CDCl 3 ):2.35(s,3H,-COCH 3 ),3.41(s,3H,-CH 3 ), 6.83-7.75 (m, 7H, Ar-H).
Embodiment 2
[0026] In the first step, add 1 mol of 10-methylphenothiazine, 7.5 mol of acetic anhydride and 2.5 mol of phosphoric acid into a dry three-necked flask, and react at 50°C for 1 hour to obtain a reaction solution;
[0027] The second step is to cool the reaction liquid to room temperature, pour it into 10mL water, filter after the solid is precipitated, wash the filter cake with water, and dry it with a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:5. Recrystallize the filter cake to obtain 3-acetyl-10-methylphenothiazine with a yield of 90.0%.
Embodiment 3
[0029] In the first step, add 1 mol of 10-methylphenothiazine, 8.9 mol of acetic anhydride and 2.5 mol of phosphoric acid into a dry three-necked flask, and react at 60°C for 1.5 hours to obtain a reaction solution;
[0030] The second step is to cool the reaction solution to room temperature, pour it into 12mL water, filter after the solid is precipitated, wash the filter cake with water, and dry it with a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:5. Recrystallize the filter cake to obtain 3-acetyl-10-methylphenothiazine with a yield of 87.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com