Preparation of caprolactone, caprolactam, 2,5-etrahydrofuran-<wbr/>dimethanol, 1,6-<wbr/>hexanediol or 1,2,6-<wbr/>hexanetriol from 5-<wbr/>hydroxymethyl-<wbr/>2-furfuraldehyde
A technology of tetrahydrofuran and hexanediol, applied in the field of preparing ε-caprolactam, can solve the problems of hindering microbial intermediates, laborious and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1 Direct hydrogenation from HMF to 1,6-hexanediol
[0097] In a stirred 100 ml autoclave, 0.1 g of copper chromite and 0.06 g of Pd on carbon (10%) were added to a solution of 0.5 g of HMF in 25 ml of methanol. Close the lid of the autoclave, start stirring at 1000rpm, and after three vacuum / nitrogen cycles, bring the autoclave at 3MPa H 2 The pressure was lowered and the temperature was raised to 80°C. After 1.5 hours, the hydrogen pressure increased to 15Mpa, and the temperature increased to 270°C. The autoclave was continued under these conditions with stirring for an additional 14.5 hours. After cooling to room temperature, the pressure was released and GC analysis of the contents of the autoclave showed the presence of 4.2% 1,6-hexanediol and 2.3% 1,2,6-hexanetriol.
Embodiment 2~15
[0098] Examples 2-15 Hydrogenation from HMF to THFDM
[0099] In a stirred 100 ml autoclave, 0.05 g of 5 mol% Ru / C (Aldrich) was added to a solution of 0.5 g of HMF in 30 ml of methanol. Close the lid of the autoclave, start stirring at 1000rpm, and after three vacuum / nitrogen cycles, bring the autoclave at 5MPa H 2 The pressure was lowered and the temperature increased to 75°C. After 1.5 hours, the hydrogen pressure was raised to 9 MPa, and the temperature was raised to 200°C. The autoclave was continued under these conditions with stirring for an additional 14 hours. After cooling to room temperature, the pressure was released and GC analysis of the contents of the autoclave showed the presence of 30% THFDM.
[0100] In the same manner, several other catalysts were tested in this hydrogenation and the results are summarized in Table 1.
[0101] Table 1 Hydrogenation from HMF to 2,5-THF-dimethanol a
[0102] Example catalyst 2,5-THF-dimethanol% 2 Ru / ...
Embodiment 16~22
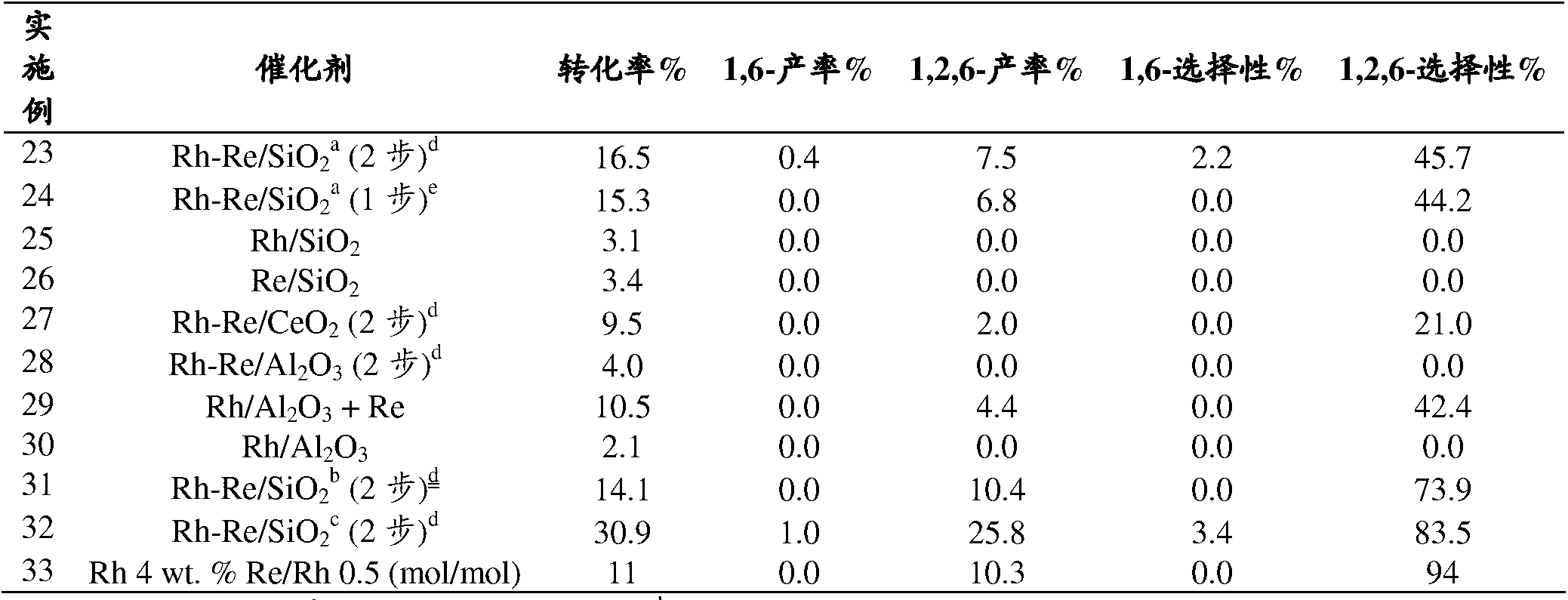
[0111] Examples 16-22: Hydrogenation from THFDM to 1,6-hexanediol
[0112] In a stirred 100 ml autoclave, 0.1 g copper chromite was added to a solution of 0.5 g THFDM in 30 ml n-propanol. Close the lid of the autoclave, start stirring at 1000rpm, and after three vacuum / nitrogen cycles, bring the autoclave under 10MPa H 2 The pressure was lowered and the temperature increased to 260°C. The autoclave was continued under these conditions with stirring for an additional 6 hours. After cooling to room temperature, the pressure was released and GC analysis of the contents of the autoclave showed the presence of 17.3% 1,6-hexanediol and 3.7% 1,2,6-hexanetriol. Other catalysts were tested under similar conditions (Table 3).
[0113] Table 3: Hydrogenation of THFDM
[0114] Example catalyst Conversion rate Yield of 1,6-hexanediol Yield of 1,2,6-hexanetriol 16 CuCr 70% 17.3% 3.7% 17 CuZn (JM PR-A) 26% 1.8% 5.4% 18 CuZn(JM PR-B) 71% 2.1% 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com